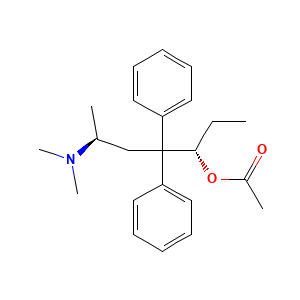
| Synonyms |
LAAM; Levacetilmetadol; Levacetylmethadol; Levacetylmethadolum; Levomethadyl; Orlaam; Levacetylmethadol [INN]; Levacetilmetadol [INN-Spanish]; Levacetylmethadol (INN); Levacetylmethadolum [INN-Latin]; Levo-Alphacetylmethadol; Levo-Methadyl acetate; Levomethadyl acetate (USAN); Orlaam (TN); A-l-acetylmethadol; Alpha-l-Acetylmethadol; L-alpha-Acetylmethadol; Levo-alpha-Acetylmethadol; N-alpha-Acetylmethadol; Alpha-(-)-Acetylmethadol; [(3S,6S)-6-(dimethylamino)-4,4-diphenylheptan-3-yl] acetate; [S-(R*,R*)]-beta-[2-Dimethylamino)propyl]-alpha-ethyl-beta-phenylbenzeneethanol acetate (ester); Benzeneethanol, beta-[(2S)-2-(dimethylamino)propyl]-alpha-ethyl-beta-phenyl-, acetate (ester), (alphaS)-(9CI); (-)-alpha-Acetylmethadol; (1S,4S)-4-(dimethylamino)-1-ethyl-2,2-diphenylpentyl acetate; (3S,6S)-6-(dimethylamino)-4,4-diphenylheptan-2-yl acetate; 1-alpha-Acetylmethadol; 3-Heptanol, 6-(dimethylamino)-4,4-diphenyl-, acetate (ester), (3S,6S)-(-)-(8CI)
|
| Cross-matching ID |
- PubChem CID
- 15130
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05PIZ
- Formula
- C23H31NO2
- Canonical SMILES
- CC[C@@H](C(C[C@H](C)N(C)C)(C1=CC=CC=C1)C2=CC=CC=C2)OC(=O)C
- InChI
- InChI=1S/C23H31NO2/c1-6-22(26-19(3)25)23(17-18(2)24(4)5,20-13-9-7-10-14-20)21-15-11-8-12-16-21/h7-16,18,22H,6,17H2,1-5H3/t18-,22-/m0/s1
- InChIKey
- XBMIVRRWGCYBTQ-AVRDEDQJSA-N
|