| Synonyms |
Talavir; VACV; ValACV; Valacyclovir; Virval; Zelitrex; Valaciclovir Hcl; Valacyclover Hydrochloric; Valacyclover Hydrochloride; BW256U87; TBB067866; Acyclovir-valine; BW-256U; Valaciclovir (INN); Valaciclovir [INN:BAN]; Valaciclovir, Valtrex; Valtrex (TN); Zelitrex (TN); Valacyclovir, (L)-isomer; L-Valine ester with 9-((2-hydroxyethoxy)methyl)guanine; L-Valine, 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)ethyl ester; L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9 H-purin-9-yl)methoxy]ethyl ester, monohydrochloride; 2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl L-valinate; 2-[(2-amino-6-oxo-3H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate; 2-{[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methyl]oxy}ethyl L-valinate
|
| Cross-matching ID |
- PubChem CID
- 135398742
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04QJD
- Formula
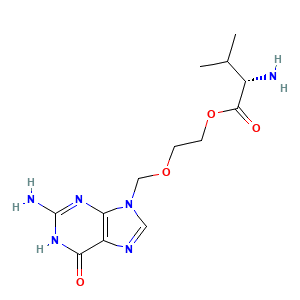
- C13H20N6O4
- Canonical SMILES
- CC(C)[C@@H](C(=O)OCCOCN1C=NC2=C1N=C(NC2=O)N)N
- InChI
- InChI=1S/C13H20N6O4/c1-7(2)8(14)12(21)23-4-3-22-6-19-5-16-9-10(19)17-13(15)18-11(9)20/h5,7-8H,3-4,6,14H2,1-2H3,(H3,15,17,18,20)/t8-/m0/s1
- InChIKey
- HDOVUKNUBWVHOX-QMMMGPOBSA-N
|