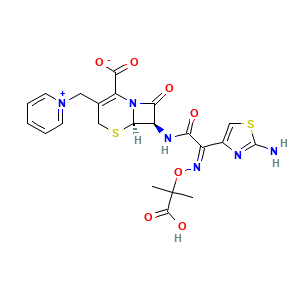
| Synonyms |
Ceftazidima; Ceftazidimum; Ceptaz; Fortaz; Ceftazidime Sodium In Plastic Container; Ceftazidime anhydrous; Ceftazidime pentahydrate; Fortaz In Plastic Container; SN 401; CEFTAZIDIME (ARGININE FORMULATION); Ceftazidima [INN-Spanish]; Ceftazidime (INN); Ceftazidime (TN); Ceftazidimum [INN-Latin]; Cefzim (TN); Ceptaz (TN); Fortaz (TN); Fortum (TN); (6R,7R)-7-({(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(1-carboxy-1-methylethoxy)imino]acetyl}amino)-8-oxo-3-(pyridinium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; (6R,7R)-7-[[(2E)-2-(2-amino-1,3-thiazol-4-yl)-2-(1-hydroxy-2-methyl-1-oxopropan-2-yl)oxyiminoacetyl]amino]-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(1-hydroxy-2-methyl-1-oxopropan-2-yl)oxyiminoacetyl]amino]-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; (6R,7R)-7-[[2-(2-amino-1,3-thiazol-4-yl)-2-(1-hydroxy-2-methyl-1-oxopropan-2-yl)oxyiminoacetyl]amino]-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(2-carboxypropan-2-yl)oxy]imino}acetyl]amino}-8-oxo-3-(pyridinium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; 7-[[2-(2-amino-1,3-thiazol-4-yl)-2-(1-hydroxy-2-methyl-1-oxopropan-2-yl)oxyiminoacetyl]amino]-8-oxo-3-(pyridin-1-ium-1-ylmethyl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; 7beta-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(2-carboxypropan-2-yl)oxy]imino}acetyl]amino}-3-(pyridinium-1-ylmethyl)-3,4-didehydrocepham-4-carboxylate
|
| Cross-matching ID |
- PubChem CID
- 5481173
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0PH5Z
- Formula
- C22H22N6O7S2
- Canonical SMILES
- CC(C)(C(=O)O)O/N=C(/C1=CSC(=N1)N)\\C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C[N+]4=CC=CC=C4)C(=O)[O-]
- InChI
- InChI=1S/C22H22N6O7S2/c1-22(2,20(33)34)35-26-13(12-10-37-21(23)24-12)16(29)25-14-17(30)28-15(19(31)32)11(9-36-18(14)28)8-27-6-4-3-5-7-27/h3-7,10,14,18H,8-9H2,1-2H3,(H4-,23,24,25,29,31,32,33,34)/b26-13-/t14-,18-/m1/s1
- InChIKey
- ORFOPKXBNMVMKC-DWVKKRMSSA-N
|