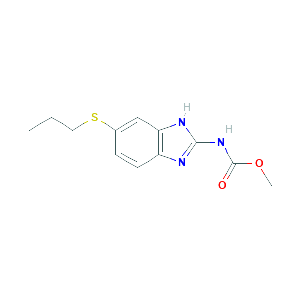
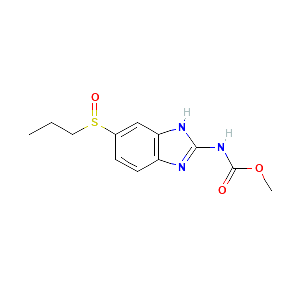
| References |
| 1 |
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY:McGraw-Hill, 2001., p. 1126
|
| 2 |
Nanocrystal Suspensions for Enhancing the Oral Absorption of Albendazole. Nanomaterials (Basel). 2022 Sep 1;12(17):3032. doi: 10.3390/nano12173032.
|
| 3 |
Multi-residue methodology for quantification of antiparasitics in hen eggs by LC-MS/MS: development, validation and application to 348 samples from Brazil. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2022 Aug;39(8):1412-1423. doi: 10.1080/19440049.2022.2093984.
|
| 4 |
Improvement of Albendazole Bioavailability with Menbutone Administration in Sheep. Animals (Basel). 2022 Feb 14;12(4):463. doi: 10.3390/ani12040463.
|
| 5 |
Effect of clotrimazole on microsomal metabolism and pharmacokinetics of albendazole J Pharm Pharmacol. 2003 Jun;55(6):757-64. doi: 10.1211/002235703765951357.
|
| 6 |
CYP2J2 and CYP2C19 are the major enzymes responsible for metabolism of albendazole and fenbendazole in human liver microsomes and recombinant P450 assay systems. Antimicrob Agents Chemother. 2013 Nov;57(11):5448-56.
|
| 7 |
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; Albendazole, Summary Report(3). EMEA/MRL/865/03-Final (June 2004). Available from, as of July 25, 2006:
|
| 8 |
Secretion into Milk of the Main Metabolites of the Anthelmintic Albendazole Is Mediated by the ABCG2/BCRP Transporter. Antimicrob Agents Chemother. 2022 Jul 19;66(7):e0006222. doi: 10.1128/aac.00062-22.
|