Details of Drug-Metabolizing Enzyme (DME)
| Full List of Drug(s) Metabolized by This DME | |||||
|---|---|---|---|---|---|
| Drugs Approved by FDA | Click to Show/Hide the Full List of Drugs: 719 Drugs | ||||
Cobicistat |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [1] |
Darolutamide |
Drug Info | Approved | Prostate cancer | ICD11: 2C82 | [2] |
Bupropion |
Drug Info | Approved | Nicotine dependence | ICD11: 6C4A | [3] |
Tamoxifen citrate |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [4] |
Yn-968D1 |
Drug Info | Approved | Breast cancer | ICD11: 2C60-2C6Y | [5] |
Erlotinib hydrochloride |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [6] |
Alosetron hydrochloride |
Drug Info | Approved | Irritable bowel syndrome | ICD11: DD91 | [7] |
Arbidol |
Drug Info | Approved | Virus infection | ICD11: 1A24-1D9Z | [8] |
Fostemsavir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60-1C62 | [9] |
Fostemsavir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60-1C62 | [10] |
Idelalisib |
Drug Info | Approved | Chronic lymphocytic leukaemia | ICD11: 2A82 | [11] |
Warfarin sodium |
Drug Info | Approved | Atrial fibrillation | ICD11: BC81 | [12] |
Estradiol acetate |
Drug Info | Approved | Acquired prion disease | ICD11: 8E01 | [13] |
Estradiol cypionate |
Drug Info | Approved | Acquired prion disease | ICD11: 8E01 | [13] |
Estradiol valerate |
Drug Info | Approved | Acquired prion disease | ICD11: 8E01 | [13] |
Estrone |
Drug Info | Approved | Menopausal disorder | ICD11: GA30 | [14] |
Nicotine |
Drug Info | Approved | Nicotine dependence | ICD11: 6C4A | [15] |
Apremilast |
Drug Info | Approved | Psoriasis vulgaris | ICD11: EA90 | [16] |
Atazanavir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [17] |
Cocaine |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [18] |
Palbociclib |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [19] |
Pimavanserin |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [20] |
Apalutamide |
Drug Info | Approved | Acute myeloid leukaemia | ICD11: 2A60 | [21] |
Capmatinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [22] |
Capmatinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [23] |
Dronabinol |
Drug Info | Approved | Anorexia nervosa | ICD11: 6B80 | [24] |
Ozanimod |
Drug Info | Approved | Multiple sclerosis | ICD11: 8A40 | [25] |
Betamethasone |
Drug Info | Approved | Inflammation | ICD11: 1A00-CA43 | [26] |
Bortezomib |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [27] |
Daclatasvir dihydrochloride |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [28] |
Delavirdine mesylate |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [29] |
Diclofenac sodium |
Drug Info | Approved | Osteoarthritis | ICD11: FA00 | [30] |
Hydrocodone |
Drug Info | Approved | Pain | ICD11: MG30-MG3Z | [31] |
Prednisolone |
Drug Info | Approved | Multiple myeloma | ICD11: 2A83 | [32] |
Prednisone |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [32] |
Amlodipine besylate |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [33] |
Amlodipine maleate |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [33] |
Galantamine hydrobromide |
Drug Info | Approved | Alzheimer disease | ICD11: 8A20 | [34] |
Ibrutinib |
Drug Info | Approved | Mantle cell lymphoma | ICD11: 2A85 | [35] |
Ketoconazole |
Drug Info | Approved | Dermatophytosis | ICD11: 1F28 | [36] |
Loxoprofen gel |
Drug Info | Approved | Inflammation | ICD11: 1A00-CA43 | [37] |
Metoclopramide hydrochloride |
Drug Info | Approved | Functional nausea/vomiting | ICD11: DD90 | [38] |
Montelukast sodium |
Drug Info | Approved | Asthma | ICD11: CA23 | [24] |
Naloxone |
Drug Info | Approved | Opiate dependence | ICD11: 6C43 | [39] |
Primaquine |
Drug Info | Approved | Malaria | ICD11: 1F40 | [40] |
Progesterone |
Drug Info | Approved | Premature labour | ICD11: JB00 | [41] |
Rosiglitazone |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [42] |
Acalabrutinib |
Drug Info | Approved | Mantle cell lymphoma | ICD11: 2A85 | [43] |
Almogran |
Drug Info | Approved | Migraine | ICD11: 8A80 | [44] |
Almogran |
Drug Info | Approved | Migraine | ICD11: 8A80 | [45], [46] |
Crizotinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [47] |
Imatinib mesylate |
Drug Info | Approved | Chronic myelogenous leukaemia | ICD11: 2A20 | [48] |
Lidocaine |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [49] |
Linagliptin |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [50] |
Linagliptin |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [51] |
Metronidazole |
Drug Info | Approved | Amebiasis | ICD11: 1A36 | [52] |
Temsirolimus |
Drug Info | Approved | Renal cell carcinoma | ICD11: 2C90 | [53] |
Valdecoxib |
Drug Info | Approved | Osteoarthritis | ICD11: FA00 | [54] |
Brivaracetam |
Drug Info | Approved | Focal seizure | ICD11: 8A68 | [55] |
Cannabidiol |
Drug Info | Approved | Lennox-Gastaut syndrome | ICD11: 8A62 | [56] |
Capsaicin |
Drug Info | Approved | Herpes zoster | ICD11: 1E91 | [57] |
Ciclosporin |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [58] |
Ciclosporin |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [59] |
Clomipramine hydrochloride |
Drug Info | Approved | Obsessive-compulsive disorder | ICD11: 6B20 | [60] |
Doravirine |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [61] |
Doxazosin |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [62] |
Ethinyl estradiol |
Drug Info | Approved | Menopausal disorder | ICD11: GA30 | [63] |
Fedratinib |
Drug Info | Approved | Chronic myelogenous leukaemia | ICD11: 2A20 | [64], [65] |
Ifosfamide |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [24] |
Irbesartan |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [66] |
Lapatinib ditosylate |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [67] |
Lenvatinib mesylate |
Drug Info | Approved | Thyroid cancer | ICD11: 2D10 | [68] |
Mycophenolate mofetil |
Drug Info | Approved | Crohn disease | ICD11: DD70 | [69] |
Pitavastatin |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [70] |
Silodosin |
Drug Info | Approved | Prostatic hyperplasia | ICD11: GA90 | [71] |
Siponimod |
Drug Info | Approved | Multiple sclerosis | ICD11: 8A40 | [72] |
Sunitinib malate |
Drug Info | Approved | Renal cell carcinoma | ICD11: 2C90 | [6] |
Sunitinib malate |
Drug Info | Approved | Renal cell carcinoma | ICD11: 2C90 | [73] |
Tacrolimus |
Drug Info | Approved | Ulcerative colitis | ICD11: DD71 | [18] |
Terbinafine hydrochloride |
Drug Info | Approved | Pityriasis versicolor | ICD11: 1F2D | [24] |
Ticlopidine |
Drug Info | Approved | Cerebral stroke | ICD11: 8B11 | [24] |
Tipranavir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [74] |
Tretinoin |
Drug Info | Approved | Acne vulgaris | ICD11: ED80 | [75] |
Almotriptan malate |
Drug Info | Approved | Migraine | ICD11: 8A80 | [45] |
Aprepitant |
Drug Info | Approved | Functional nausea/vomiting | ICD11: DD90 | [76] |
Asenapine maleate |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [77] |
Brentuximab vedotin |
Drug Info | Approved | Anaplastic large cell lymphoma | ICD11: 2A90 | [78] |
Bupivacaine hydrochloride |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [79] |
Cabazitaxel |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [80] |
Clomipramine |
Drug Info | Approved | Depression | ICD11: 6A70-6A7Z | [81] |
Clomipramine |
Drug Info | Approved | Depression | ICD11: 6A70-6A7Z | [82] |
Colchicine |
Drug Info | Approved | Uncontrolled gout | ICD11: FA25 | [83] |
Conjugated estrogens |
Drug Info | Approved | Menopausal disorder | ICD11: GA30 | [84] |
Cyclobenzaprine |
Drug Info | Approved | Depression | ICD11: 6A71 | [85] |
Daunorubicin |
Drug Info | Approved | Acute myeloid leukaemia | ICD11: 2A60 | [86] |
Disulfiram |
Drug Info | Approved | Alcohol dependence | ICD11: 6C40 | [87] |
Enzalutamide |
Drug Info | Approved | Prostate cancer | ICD11: 2C82 | [88] |
Enzalutamide |
Drug Info | Approved | Prostate cancer | ICD11: 2C82 | [89] |
Guanfacine hydrochloride |
Drug Info | Approved | Attention deficit hyperactivity disorder | ICD11: 6A05 | [90] |
Methyltestosterone |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [91] |
Mirabegron |
Drug Info | Approved | Overactive bladder | ICD11: GC50 | [92] |
Norethindrone |
Drug Info | Approved | Solid tumour/cancer | ICD11: 2A00-2F9Z | [93] |
Oxycodone hydrochloride |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [94] |
Oxymorphone |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [95] |
Pioglitazone hydrochloride |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [96] |
Pravastatin |
Drug Info | Approved | Dyslipidaemia | ICD11: 5C81 | [97] |
Ranolazine |
Drug Info | Approved | Angina pectoris | ICD11: BA40 | [98] |
Rivaroxaban |
Drug Info | Approved | Deep vein thrombosis | ICD11: BD71 | [99] |
Sirolimus |
Drug Info | Approved | Lymphangioleiomyomatosis | ICD11: CB07 | [18] |
Tacrine hydrochloride |
Drug Info | Approved | Alzheimer disease | ICD11: 8A20 | [44] |
Topiramate |
Drug Info | Approved | Focal seizure | ICD11: 8A68 | [100] |
Trabectedin |
Drug Info | Approved | Leiomyosarcoma | ICD11: 2B58 | [101] |
Albendazole |
Drug Info | Approved | Giardiasis | ICD11: 1A31 | [40] |
Alpelisib |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [102] |
Apomorphine |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [103] |
Beclomethasone dipropionate |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [104] |
Beclomethasone dipropionate |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [105] |
Chenodeoxycholic acid |
Drug Info | Approved | Cholelithiasis | ICD11: DC11 | [106] |
Clomiphene citrate |
Drug Info | Approved | Female infertility | ICD11: GA31 | [107] |
Copanlisib |
Drug Info | Approved | Follicular lymphoma | ICD11: 2A80 | [108] |
Deflazacort |
Drug Info | Approved | Muscular dystrophy | ICD11: 8C70 | [109] |
Dehydroepiandrosterone |
Drug Info | Approved | Dyspareunia | ICD11: GA12 | [41] |
Deoxycholic acid |
Drug Info | Approved | Obesity | ICD11: 5B81 | [110] |
Edoxaban |
Drug Info | Approved | Atrial fibrillation | ICD11: BC81 | [111] |
Etonogestrel |
Drug Info | Approved | Contraception | ICD11: JA65 | [112] |
Halothane |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [113] |
Hydrocodone bitartrate |
Drug Info | Approved | Neuropathic pain | ICD11: 8E43 | [114] |
Hydrocortisone |
Drug Info | Approved | Uncontrolled gout | ICD11: FA25 | [115] |
Indinavir sulfate |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [24] |
Ketamine |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [116] |
Levocetirizine |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [117] |
Medrysone |
Drug Info | Approved | Eyelid inflammation | ICD11: 9A02 | [118] |
Meperidine |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [119] |
Methylprednisolone |
Drug Info | Approved | Lymphoblastic Lymphoma | ICD11: 2A70 | [120] |
Nilotinib |
Drug Info | Approved | Chronic myelogenous leukaemia | ICD11: 2A20 | [121] |
Osimertinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [122] |
Osimertinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [123] |
Panobinostat lactate |
Drug Info | Approved | Multiple myeloma | ICD11: 2A83 | [124] |
Perampanel |
Drug Info | Approved | Focal seizure | ICD11: 8A68 | [125] |
Ponesimod |
Drug Info | Approved | Multiple sclerosis | ICD11: 8A40 | [126] |
Ponesimod |
Drug Info | Approved | Multiple sclerosis | ICD11: 8A40 | [127] |
Quinine sulfate |
Drug Info | Approved | Malaria | ICD11: 1F40 | [128] |
Regorafenib |
Drug Info | Approved | Colon cancer | ICD11: 2B90 | [129] |
Regorafenib |
Drug Info | Approved | Colon cancer | ICD11: 2B90 | [130] |
Remdesivir |
Drug Info | Approved | Coronavirus Disease 2019 (COVID-19) | ICD11: 1D6Y | [131] |
Tamsulosin |
Drug Info | Approved | Prostatic hyperplasia | ICD11: GA90 | [132] |
Tamsulosin |
Drug Info | Approved | Prostatic hyperplasia | ICD11: GA90 | [133] |
Telithromycin |
Drug Info | Approved | Bronchitis | ICD11: CA20 | [134] |
Teniposide |
Drug Info | Approved | Acute lymphoblastic leukemia | ICD11: 2B33 | [135] |
Tepotinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [136] |
Tepotinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [137] |
Ticagrelor |
Drug Info | Approved | Cerebral stroke | ICD11: 8B11 | [138] |
Tucatinib |
Drug Info | Approved | HER2-positive breast cancer | ICD11: 2C60-2C6Y | [139] |
Alfentanil |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [140] |
Aliskiren |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [141] |
Alitretinoin |
Drug Info | Approved | Kaposi sarcoma | ICD11: 2B57 | [142] |
Arteether |
Drug Info | Approved | Malaria | ICD11: 1F40-1F45 | [143] |
Axitinib |
Drug Info | Approved | Renal cell carcinoma | ICD11: 2C90 | [144] |
Benzphetamine |
Drug Info | Approved | Obesity | ICD11: 5B81 | [145] |
Brexpiprazole |
Drug Info | Approved | Bipolar disorder | ICD11: 6A60 | [146] |
Caffeine |
Drug Info | Approved | Orthostatic hypotension | ICD11: BA21 | [147] |
Carvedilol |
Drug Info | Approved | Congestive heart failure | ICD11: BD10 | [148] |
Cisapride |
Drug Info | Approved | Gastro-oesophageal reflux disease | ICD11: DA22 | [149] |
Dextromethorphan hydrobromide |
Drug Info | Approved | Atherosclerosis | ICD11: BA80 | [150] |
Diazepam |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [151] |
Diethylstilbestrol |
Drug Info | Approved | Vaginitis | ICD11: GA02 | [152] |
Digitoxin |
Drug Info | Approved | Congestive heart failure | ICD11: BD10 | [153] |
Dihydroergotamine |
Drug Info | Approved | Migraine | ICD11: 8A80 | [31] |
Diphenylpyraline |
Drug Info | Approved | Allergy | ICD11: 4A80 | [154] |
Doxepin hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [155] |
Drospirenone |
Drug Info | Approved | Contraception | ICD11: JA65 | [156] |
Eliglustat tartrate |
Drug Info | Approved | Gaucher disease | ICD11: 5C56 | [24] |
Ergocalciferol |
Drug Info | Approved | Hypoparathyroidism | ICD11: 5A50 | [157] |
Finasteride |
Drug Info | Approved | Prostatic hyperplasia | ICD11: GA90 | [18] |
Fluoxymesterone |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [158] |
Flutamide |
Drug Info | Approved | Prostate cancer | ICD11: 2C82 | [159] |
Gilteritinib |
Drug Info | Approved | Acute myeloid leukaemia | ICD11: 2A60 | [160] |
Gilteritinib |
Drug Info | Approved | Acute myeloid leukaemia | ICD11: 2A60 | [161] |
Haloperidol decanoate |
Drug Info | Approved | Agitation/aggression | ICD11: 6D86 | [162] |
Lansoprazole |
Drug Info | Approved | Duodenal ulcer | ICD11: DA63 | [163] |
Lasofoxifene |
Drug Info | Approved | Osteoporosis | ICD11: FB83 | [164] |
Lasofoxifene |
Drug Info | Approved | Osteoporosis | ICD11: FB83 | [165] |
Levonorgestrel |
Drug Info | Approved | Menorrhagia | ICD11: GA20 | [166] |
Levonorgestrel |
Drug Info | Approved | Menorrhagia | ICD11: GA20 | [167] |
Lorcaserin |
Drug Info | Approved | Obesity | ICD11: 5B81 | [168] |
Macitentan |
Drug Info | Approved | Pulmonary hypertension | ICD11: BB01 | [169] |
Nateglinide |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [170] |
Nevirapine |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [171] |
Norethindrone acetate |
Drug Info | Approved | Menorrhagia | ICD11: GA20 | [93] |
Olaparib |
Drug Info | Approved | Ovarian cancer | ICD11: 2C73 | [172] |
Ondansetron |
Drug Info | Approved | Gastritis | ICD11: DA42 | [173] |
Pomalidomide |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [174] |
Propafenone hydrochloride |
Drug Info | Approved | Ventricular tachyarrhythmia | ICD11: BC71 | [175] |
Propranolol hydrochloride |
Drug Info | Approved | Migraine | ICD11: 8A80 | [176] |
Repaglinide |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [170] |
Rifabutin |
Drug Info | Approved | Pulmonary tuberculosis | ICD11: 1B10 | [177] |
Rofecoxib |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [24] |
Rotigotine |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [178] |
SR141716A |
Drug Info | Approved | Obesity | ICD11: 5B80-5B81 | [179] |
Tivozanib |
Drug Info | Approved | Renal cell carcinoma | ICD11: 2C90 | [180] |
Tolcapone |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [181] |
Toremifene citrate |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [182] |
Trazodone hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [183] |
Trimethoprim |
Drug Info | Approved | Infectious cystitis | ICD11: GC00 | [18] |
Verapamil hydrochloride |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [24] |
Vilanterol |
Drug Info | Approved | Asthma | ICD11: CA23 | [184] |
Ziprasidone |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [185] |
Amitriptyline hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [186] |
Amprenavir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [187] |
Artemether |
Drug Info | Approved | Malaria | ICD11: 1F40 | [188] |
Avanafil |
Drug Info | Approved | Erectile dysfunction | ICD11: HA01 | [189] |
Baricitinib |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [190] |
Baricitinib |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [191] |
Belinostat |
Drug Info | Approved | Anaplastic large cell lymphoma | ICD11: 2A90 | [192] |
Betrixaban |
Drug Info | Approved | Venous thromboembolism | ICD11: BD72 | [193] |
Brigatinib |
Drug Info | Approved | Anaplastic large cell lymphoma | ICD11: 2A90 | [194] |
Bromfenac |
Drug Info | Approved | Cataract | ICD11: 9B10 | [195] |
Buspirone |
Drug Info | Approved | Anxiety disorder | ICD11: 6B00 | [196] |
Carbamazepine |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [197] |
Cenobamate |
Drug Info | Approved | Focal seizure | ICD11: 8A68 | [198] |
Cevimeline hydrochloride |
Drug Info | Approved | Sjogren syndrome | ICD11: 4A43 | [199] |
Cinacalcet hydrochloride |
Drug Info | Approved | Hyperparathyroidism | ICD11: 5A51 | [200] |
Clobazam |
Drug Info | Approved | Lennox-Gastaut syndrome | ICD11: 8A62 | [201] |
Codeine |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [202] |
Cortisone acetate |
Drug Info | Approved | Prostate cancer | ICD11: 2C82 | [203] |
Cyclophosphamide |
Drug Info | Approved | Multiple myeloma | ICD11: 2A83 | [204] |
Dabrafenib mesylate |
Drug Info | Approved | Melanoma | ICD11: 2C30 | [205] |
Darifenacin hydrobromide |
Drug Info | Approved | Overactive bladder | ICD11: GC50 | [206] |
Dasatinib |
Drug Info | Approved | Chronic myelogenous leukaemia | ICD11: 2A20 | [207] |
Desvenlafaxine |
Drug Info | Approved | Major depressive disorder | ICD11: 6A70-6A7Z | [208] |
Desvenlafaxine |
Drug Info | Approved | Major depressive disorder | ICD11: 6A70-6A7Z | [209], [] |
Desvenlafaxine succinate |
Drug Info | Approved | Depression | ICD11: 6A71 | [210] |
Diltiazem hydrochloride |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [211] |
Dolutegravir sodium |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [212] |
Donepezil hydrochloride |
Drug Info | Approved | Alzheimer disease | ICD11: 8A20 | [213] |
Doxorubicin |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [214] |
Dronedarone hydrochloride |
Drug Info | Approved | Atrial fibrillation | ICD11: BC81 | [215] |
Efavirenz |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [216] |
Erdafitinib |
Drug Info | Approved | Renal cell carcinoma | ICD11: 2C90 | [217] |
Exjade |
Drug Info | Approved | Hyperphosphatemia | ICD11: 5C64 | [218] |
Felbamate |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [219] |
Fluoxetine hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [220] |
Flurazepam |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [221] |
Fluvastatin sodium |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [222] |
Histamine |
Drug Info | Approved | Allergy | ICD11: 4A80 | [223] |
Ibuprofen |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [224] |
Imipramine hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [225] |
Infigratinib |
Drug Info | Approved | Discovery agent | ICD: N.A. | [226], [227] |
Infigratinib |
Drug Info | Approved | Discovery agent | ICD: N.A. | [226] |
Isotretinoin |
Drug Info | Approved | Acne vulgaris | ICD11: ED80 | [228] |
Isotretinoin |
Drug Info | Approved | Acne vulgaris | ICD11: ED80 | [229] |
Isradipine |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [18] |
Isradipine |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [230] |
Istradefylline |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [231] |
Ivermectin |
Drug Info | Approved | Onchocerciasis | ICD11: 1F6A | [232] |
Ivosidenib |
Drug Info | Approved | Acute myeloid leukaemia | ICD11: 2A60 | [233] |
Ivosidenib |
Drug Info | Approved | Acute myeloid leukaemia | ICD11: 2A60 | [234] |
Leflunomide |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [235] |
Leflunomide |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [236] |
Lesinurad |
Drug Info | Approved | Uncontrolled gout | ICD11: FA25 | [237] |
Letermovir |
Drug Info | Approved | Cytomegalovirus infection | ICD11: 1D82 | [238] |
Levomepromazine |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [239] |
Lurasidone |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [240] |
Metoprolol succinate |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [241] |
Metoprolol tartrate |
Drug Info | Approved | Angina pectoris | ICD11: BA40 | [242] |
Midazolam hydrochloride |
Drug Info | Approved | Anxiety disorder | ICD11: 6B00 | [243] |
Mometasone furoate |
Drug Info | Approved | Allergy | ICD11: 4A80 | [118] |
Morphine |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [244] |
Nefazodone |
Drug Info | Approved | Depression | ICD11: 6A71 | [245] |
Neratinib |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [246] |
Pexidartinib |
Drug Info | Approved | Neurofibromatosis | ICD11: LD2D | [247] |
Phenytoin |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [248] |
Prazepam |
Drug Info | Approved | Anxiety disorder | ICD11: 6B00 | [249] |
Promazine |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [250] |
Propoxyphene Hydrochloride |
Drug Info | Approved | Pain | ICD11: MG30-MG3Z | [251] |
Quinidine |
Drug Info | Approved | Ventricular tachyarrhythmia | ICD11: BC71 | [252] |
Rilpivirine hydrochloride |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [253] |
Ropivacaine hydrochloride |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [254] |
Rucaparib |
Drug Info | Approved | Ovarian cancer | ICD11: 2C73 | [255] |
Simvastatin |
Drug Info | Approved | Dyslipidaemia | ICD11: 5C81 | [256] |
Simvastatin |
Drug Info | Approved | Dyslipidaemia | ICD11: 5C81 | [257] |
Sulfinpyrazone |
Drug Info | Approved | Uncontrolled gout | ICD11: FA25 | [258] |
Suvorexant |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [259] |
Tofacitinib citrate |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [260] |
Vismodegib |
Drug Info | Approved | Melanoma | ICD11: 2C30 | [261] |
Zanubrutinib |
Drug Info | Approved | Mantle cell lymphoma | ICD11: 2A85 | [262] |
Zanubrutinib |
Drug Info | Approved | Mantle cell lymphoma | ICD11: 2A85 | [263] |
Zileuton |
Drug Info | Approved | Asthma | ICD11: CA23 | [24] |
Zolpidem tartrate |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [264] |
Alectinib hydrochloride |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [265] |
Amiodarone hydrochloride |
Drug Info | Approved | Ventricular tachyarrhythmia | ICD11: BC71 | [36] |
Anastrozole |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [266] |
Argatroban |
Drug Info | Approved | Thrombosis | ICD11: DB61 | [267] |
Atorvastatin calcium |
Drug Info | Approved | Dyslipidaemia | ICD11: 5C81 | [24] |
Azelastine |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [268] |
Bedaquiline fumarate |
Drug Info | Approved | Pulmonary tuberculosis | ICD11: 1B10 | [269] |
Betamethasone valerate |
Drug Info | Approved | Psoriasis vulgaris | ICD11: EA90 | [270] |
Budesonide |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [271] |
Buprenorphine hydrochloride |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [272] |
Cabozantinib |
Drug Info | Approved | Thyroid cancer | ICD11: 2D10 | [273] |
Chlorpromazine hydrochloride |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [274] |
Citalopram hydrobromide |
Drug Info | Approved | Depression | ICD11: 6A71 | [275] |
Clarithromycin |
Drug Info | Approved | Pneumocystis pneumonia | ICD11: CA40 | [276] |
Clofazimine |
Drug Info | Approved | Crohn disease | ICD11: DD70 | [18] |
Clonidine |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [277] |
Clozapine |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [278] |
Delamanid |
Drug Info | Approved | Pulmonary tuberculosis | ICD11: 1B10 | [279] |
Dofetilide |
Drug Info | Approved | Atrial fibrillation | ICD11: BC81 | [280] |
Dolasetron mesylate |
Drug Info | Approved | Functional nausea/vomiting | ICD11: DD90 | [281] |
Doxapram hydrochloride |
Drug Info | Approved | Chronic obstructive pulmonary disease | ICD11: CA22 | [282] |
Entrectinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [283] |
Eszopiclone |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [24] |
Ethosuximide |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [284] |
Etravirine |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [285] |
Everolimus |
Drug Info | Approved | Renal cell carcinoma | ICD11: 2C90 | [286] |
Exemestane |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [287] |
Famciclovir |
Drug Info | Approved | Genital herpes | ICD11: 1A94 | [288] |
Fentanyl citrate |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [289] |
Fingolimod |
Drug Info | Approved | Multiple sclerosis | ICD11: 8A40 | [290] |
Fingolimod hydrochloride |
Drug Info | Approved | Multiple sclerosis | ICD11: 8A40 | [290] |
Flunisolide |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [291] |
Fosphenytoin sodium |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [292] |
Fostamatinib |
Drug Info | Approved | Thrombocytopenia | ICD11: 3B64 | [293] |
Gemfibrozil |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [294] |
Glipizide |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [295] |
Indapamide |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [296] |
Intedanib |
Drug Info | Approved | Idiopathic pulmonary fibrosis | ICD11: CB03 | [297] |
Isosorbide dinitrate |
Drug Info | Approved | Anal fissure | ICD11: DB50 | [298] |
Isosorbide mononitrate |
Drug Info | Approved | Angina pectoris | ICD11: BA40 | [298] |
Ivabradine hydrochloride |
Drug Info | Approved | Acute coronary syndrome | ICD11: BA4Z | [299] |
Ixabepilone |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [300] |
Levobupivacaine |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [301] |
Levorphanol |
Drug Info | Approved | Pain | ICD11: MG30-MG3Z | [302] |
Levothyroxine sodium |
Drug Info | Approved | Hypothyroidism | ICD11: 5A00 | [303] |
Loxapine succinate |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [304] |
Lurbinectedin |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [305] |
Mifepristone |
Drug Info | Approved | Cushing syndrome | ICD11: 5A70 | [306] |
Mirtazapine |
Drug Info | Approved | Depression | ICD11: 6A71 | [307] |
Nalmefene |
Drug Info | Approved | Opiate dependence | ICD11: 6C43 | [308] |
Naloxegol oxalate |
Drug Info | Approved | Opioid-induced constipation | ICD11: DB32 | [309] |
Nisoldipine |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [18] |
Omeprazole |
Drug Info | Approved | Gastro-oesophageal reflux disease | ICD11: DA22 | [24] |
Ospemifene |
Drug Info | Approved | Dyspareunia | ICD11: GA12 | [310] |
Oxycodone |
Drug Info | Approved | Pain | ICD11: MG30-MG3Z | [311] |
Paroxetine hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [312] |
Pazopanib |
Drug Info | Approved | Renal cell carcinoma | ICD11: 2C90 | [313] |
Perphenazine |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [314] |
Phenprocoumon |
Drug Info | Approved | Thrombosis | ICD11: DB61 | [315] |
Pimozide |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [316] |
Ponatinib hydrochloride |
Drug Info | Approved | Acute lymphoblastic leukemia | ICD11: 2B33 | [317] |
Pralsetinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [318] |
Propofol |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [319] |
Quetiapine fumarate |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [320] |
Rabeprazole sodium |
Drug Info | Approved | Gastro-oesophageal reflux disease | ICD11: DA22 | [321] |
Raloxifene |
Drug Info | Approved | Osteoporosis | ICD11: FB83 | [322] |
Ritonavir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [323] |
Ropinirole hydrochloride |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [324] |
Saquinavir mesylate |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [325] |
Solifenacin succinate |
Drug Info | Approved | Overactive bladder | ICD11: GC50 | [326] |
Sulfamethoxazole |
Drug Info | Approved | Infectious cystitis | ICD11: GC00 | [327] |
Tandospirone |
Drug Info | Approved | Anxiety disorder | ICD11: 6B00-6B0Z | [328] |
Tasimelteon |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [329] |
Telaprevir |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [330] |
Theophylline |
Drug Info | Approved | Chronic obstructive pulmonary disease | ICD11: CA22 | [331] |
Thioridazine |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [332] |
Thiotepa |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [333] |
Tramadol hydrochloride |
Drug Info | Approved | Neuropathic pain | ICD11: 8E43 | [334] |
Triazolam |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [335] |
Vemurafenib |
Drug Info | Approved | Melanoma | ICD11: 2C30 | [336] |
Venlafaxine hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [24] |
VESNARINONE |
Drug Info | Approved | Cardiac failure | ICD11: BD10-BD1Z | [337] |
Vilazodone hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [338] |
Vortioxetine hydrobromide |
Drug Info | Approved | Depression | ICD11: 6A71 | [339] |
Zafirlukast |
Drug Info | Approved | Asthma | ICD11: CA23 | [340] |
Acetaminophen |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [341] |
Alfuzosin hydrochloride |
Drug Info | Approved | Prostatic hyperplasia | ICD11: GA90 | [342] |
Alprazolam |
Drug Info | Approved | Anxiety disorder | ICD11: 6B00 | [343] |
Apixaban |
Drug Info | Approved | Deep vein thrombosis | ICD11: BD71 | [344] |
Aripiprazole lauroxil |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [345] |
Avapritinib |
Drug Info | Approved | Gastrointestinal stromal tumour | ICD11: 2B5B | [346] |
Avapritinib |
Drug Info | Approved | Gastrointestinal stromal tumour | ICD11: 2B5B | [347] |
Binimetinib |
Drug Info | Approved | Melanoma | ICD11: 2C30 | [348] |
Calcitriol |
Drug Info | Approved | Congenital alopecia | ICD11: LC30 | [349] |
Canagliflozin |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [350] |
Chlordiazepoxide |
Drug Info | Approved | Anxiety disorder | ICD11: 6B00-6B0Z | [351] |
Chlorpheniramine |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [53] |
Ciclesonide |
Drug Info | Approved | Asthma | ICD11: CA23 | [352] |
Cisplatin |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [353] |
Clonazepam |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [145] |
Dapagliflozin |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [354] |
Darunavir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [355] |
Desloratadine |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [356] |
Dexamethasone sodium phosphate |
Drug Info | Approved | Ataxia-telangiectasia | ICD11: 4A01 | [357] |
Dexlansoprazole |
Drug Info | Approved | Gastro-oesophageal reflux disease | ICD11: DA22 | [358] |
Digoxin |
Drug Info | Approved | Cardiac arrhythmia | ICD11: BC65 | [359] |
Docetaxel |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [360] |
Dutasteride |
Drug Info | Approved | Prostatic hyperplasia | ICD11: GA90 | [361] |
Dutasteride |
Drug Info | Approved | Prostatic hyperplasia | ICD11: GA90 | [362] |
Eravacycline |
Drug Info | Approved | Methicillin-resistant staphylococcus infection | ICD11: 1D01 | [363] |
Estazolam |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [364] |
Etoposide |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [365] |
Ezetimibe |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [366] |
Fedratinib hydrochloride |
Drug Info | Approved | Myelofibrosis | ICD11: 2A22 | [367] |
Fenofibrate |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [294] |
Fesoterodine |
Drug Info | Approved | Overactive bladder | ICD11: GC50 | [368] |
Gefitinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [369] |
Grepafloxacin |
Drug Info | Approved | Bronchitis | ICD11: CA20 | [370] |
Iloperidone |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [371] |
Irinotecan hydrochloride |
Drug Info | Approved | Colon cancer | ICD11: 2B90 | [372] |
Itraconazole |
Drug Info | Approved | Blastomycosis | ICD11: 1F22 | [373] |
Lenvatinib |
Drug Info | Approved | Thyroid cancer | ICD11: 2D10 | [374] |
Levallorphan |
Drug Info | Approved | Narcotic depression | ICD11: 6A70-6A7Z | [302] |
Levomethadyl acetate hydrochloride |
Drug Info | Approved | Opiate dependence | ICD11: 6C43 | [375] |
Levomilnacipran |
Drug Info | Approved | Depression | ICD11: 6A71 | [376] |
Loperamide hydrochloride |
Drug Info | Approved | Irritable bowel syndrome | ICD11: DD91 | [377] |
Losartan potassium |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [18] |
Lovastatin |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [378] |
Macimorelin |
Drug Info | Approved | Growth hormone deficiency | ICD11: 5A60-5A61 | [379] |
Medroxyprogesterone acetate |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [380] |
Methadone |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [381] |
Mexiletine hydrochloride |
Drug Info | Approved | Ventricular tachyarrhythmia | ICD11: BC71 | [382] |
Midostaurin |
Drug Info | Approved | Mastocytosis | ICD11: 2A21 | [383] |
Mycophenolic acid |
Drug Info | Approved | Crohn disease | ICD11: DD70 | [384], [385] |
Mycophenolic acid |
Drug Info | Approved | Crohn disease | ICD11: DD70 | [386] |
Nelfinavir mesylate |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [24] |
Nimodipine |
Drug Info | Approved | Cerebral vasospasm | ICD11: BA85 | [18] |
Olopatadine |
Drug Info | Approved | Conjunctivitis | ICD11: 9A60 | [387] |
Paclitaxel |
Drug Info | Approved | Ovarian cancer | ICD11: 2C73 | [388] |
Paclitaxel |
Drug Info | Approved | Ovarian cancer | ICD11: 2C73 | [389] |
Palonosetron hydrochloride |
Drug Info | Approved | Functional nausea/vomiting | ICD11: DD90 | [390] |
Pantoprazole sodium |
Drug Info | Approved | Gastro-oesophageal reflux disease | ICD11: DA22 | [391] |
Pergolide mesylate |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [178] |
Phenelzine |
Drug Info | Approved | Depression | ICD11: 6A71 | [392] |
Phenobarbital |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [393] |
Pinacidil |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [394] |
Pitavastatin calcium |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [395] |
Pitolisant |
Drug Info | Approved | Excessive daytime sleepiness | ICD11: MG42 | [396] |
Rifampin |
Drug Info | Approved | Tuberculosis | ICD11: 1B10-1B14 | [397] |
Riociguat |
Drug Info | Approved | Pulmonary hypertension | ICD11: BB01 | [398] |
Ruxolitinib |
Drug Info | Approved | Chronic myelogenous leukaemia | ICD11: 2A20 | [399] |
Ruxolitinib |
Drug Info | Approved | Chronic myelogenous leukaemia | ICD11: 2A20 | [400] |
Ruxolitinib phosphate |
Drug Info | Approved | Myelofibrosis | ICD11: 2A22 | [401] |
Safinamide |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [402] |
Safinamide mesylate |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [403], [404] |
Salbutamol |
Drug Info | Approved | Chronic obstructive pulmonary disease | ICD11: CA22 | [18] |
Selinexor |
Drug Info | Approved | Multiple myeloma | ICD11: 2A83 | [405] |
Sitagliptin |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [406] |
Sonidegib phosphate |
Drug Info | Approved | Basal cell carcinoma | ICD11: 2C32 | [407] |
Sorafenib |
Drug Info | Approved | Renal cell carcinoma | ICD11: 2C90 | [408] |
Sulfadiazine |
Drug Info | Approved | Toxoplasmosis | ICD11: 1F57 | [18] |
Tapinarof |
Drug Info | Approved | Discovery agent | ICD: N.A. | [409] |
Taxol |
Drug Info | Approved | Breast cancer | ICD11: 2C60-2C6Y | [410] |
Timolol maleate |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [411] |
Tolterodine tartrate |
Drug Info | Approved | Overactive bladder | ICD11: GC50 | [412] |
Troglitazone |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [413] |
Vinorelbine tartrate |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [414] |
Voriconazole |
Drug Info | Approved | Candidiasis | ICD11: 1F23 | [415] |
Zaleplon |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [416] |
Zidovudine |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [417] |
Abemaciclib |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [418] |
Abiraterone acetate |
Drug Info | Approved | Prostate cancer | ICD11: 2C82 | [419] |
Alogliptin |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [420] |
Ambrisentan |
Drug Info | Approved | Pulmonary hypertension | ICD11: BB01 | [421] |
Armodafinil |
Drug Info | Approved | Obstructive sleep apnea | ICD11: 7A41 | [422] |
Atenolol |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [423] |
Azilsartan |
Drug Info | Approved | Hypertension | ICD11: BA00-BA04 | [424], [425] |
Bicalutamide |
Drug Info | Approved | Prostate cancer | ICD11: 2C82 | [426] |
Boceprevir |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [427] |
Bosentan |
Drug Info | Approved | Pulmonary hypertension | ICD11: BB01 | [428] |
Bromocriptine mesylate |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [429] |
Busulfan |
Drug Info | Approved | Chronic myelogenous leukaemia | ICD11: 2A20 | [430] |
Cabergoline |
Drug Info | Approved | Hyperprolactinaemia | ICD11: 5A60 | [178] |
Cariprazine hydrochloride |
Drug Info | Approved | Bipolar disorder | ICD11: 6A60 | [431] |
Celecoxib |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [432] |
Cerivastatin sodium |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [24] |
Cilostazol |
Drug Info | Approved | Arteriosclerosis obliterans | ICD11: BD40 | [433] |
Clindamycin |
Drug Info | Approved | Acne vulgaris | ICD11: ED80 | [434] |
Clofibrate |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [294] |
Clopidogrel bisulfate |
Drug Info | Approved | Acute coronary syndrome | ICD11: BA4Z | [24] |
Cyproterone |
Drug Info | Approved | Prostate cancer | ICD11: 2C82 | [435] |
Cytarabine |
Drug Info | Approved | Acute lymphoblastic leukemia | ICD11: 2B33 | [436] |
Dapsone |
Drug Info | Approved | Pneumocystis pneumonia | ICD11: CA40 | [437] |
Difluprednate |
Drug Info | Approved | Anterior uveitis | ICD11: 9A96 | [438] |
Dydrogesterone |
Drug Info | Approved | Menorrhagia | ICD11: GA20 | [439] |
Eletriptan hydrobromide |
Drug Info | Approved | Migraine | ICD11: 8A80 | [24] |
Elvitegravir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [440] |
Enalapril |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [18] |
Eplerenone |
Drug Info | Approved | Ventricular dysfunction | ICD11: BD13 | [441] |
Ergotamine tartrate |
Drug Info | Approved | Migraine | ICD11: 8A80 | [442] |
Escitalopram |
Drug Info | Approved | Depression | ICD11: 6A71 | [275] |
Estramustine |
Drug Info | Approved | Prostate cancer | ICD11: 2C82 | [443] |
Estropipate |
Drug Info | Approved | Hypogonadism | ICD11: 5A61 | [444] |
Ethynodiol diacetate |
Drug Info | Approved | Contraception | ICD11: JA65 | [445] |
Flibanserin |
Drug Info | Approved | Inhibited sexual desire | ICD11: HA00 | [446] |
Fluconazole |
Drug Info | Approved | Candidiasis | ICD11: 1F23 | [447] |
Fludrocortisone |
Drug Info | Approved | Hyponatraemia | ICD11: 5C72 | [18] |
Fluorometholone |
Drug Info | Approved | Chronic obstructive pulmonary disease | ICD11: CA22 | [118] |
Fosamprenavir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [448] |
Granisetron hydrochloride |
Drug Info | Approved | Functional nausea/vomiting | ICD11: DD90 | [449] |
Hydromorphone |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [450] |
Icatibant |
Drug Info | Approved | Hereditary angioedema | ICD11: 4A00 | [451] |
Iclaprim |
Drug Info | Approved | Methicillin-resistant staphylococcus infection | ICD11: 1D01 | [452] |
Isavuconazole |
Drug Info | Approved | Allergic bronchopulmonary aspergillosis | ICD11: CA82 | [453] |
Ivacaftor |
Drug Info | Approved | Cystic fibrosis | ICD11: CA25 | [454] |
Lacosamide |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [455] |
Levomethadyl Acetate |
Drug Info | Approved | Opiate dependence | ICD11: 6C43 | [456] |
Levomethadyl Acetate |
Drug Info | Approved | Opiate dependence | ICD11: 6C43 | [457] |
Lomitapide mesylate |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [458] |
Lopinavir |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [459] |
Loratadine |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [18] |
Loteprednol etabonate |
Drug Info | Approved | Conjunctivitis | ICD11: 9A60 | [460] |
Mebendazole |
Drug Info | Approved | Ascariasis | ICD11: 1F62 | [461] |
Mefloquine hydrochloride |
Drug Info | Approved | Malaria | ICD11: 1F40 | [462] |
Megestrol acetate |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [463] |
Mephenytoin |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [464] |
Mitotane |
Drug Info | Approved | Adrenocortical carcinoma | ICD11: 2D11 | [465] |
Moxidectin |
Drug Info | Approved | Onchocerciasis | ICD11: 1F6A | [466] |
Nicardipine hydrochloride |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [467] |
Nifedipine |
Drug Info | Approved | Angina pectoris | ICD11: BA40 | [468] |
Orphenadrine citrate |
Drug Info | Approved | Myalgia | ICD11: FB56 | [469] |
Oxazepam |
Drug Info | Approved | Anxiety disorder | ICD11: 6B00 | [470] |
OZ277 |
Drug Info | Approved | Malaria | ICD11: 1F40-1F45 | [471] |
Paliperidone |
Drug Info | Approved | Bipolar disorder | ICD11: 6A60 | [472] |
Pentamidine isethionate |
Drug Info | Approved | Pneumocystis pneumonia | ICD11: CA40 | [40] |
Phentermine |
Drug Info | Approved | Obesity | ICD11: 5B81 | [473] |
Prasugrel hydrochloride |
Drug Info | Approved | Acute coronary syndrome | ICD11: BA4Z | [474] |
Pretomanid |
Drug Info | Approved | Pulmonary tuberculosis | ICD11: 1B10 | [475] |
Pyrazinamide |
Drug Info | Approved | Pulmonary tuberculosis | ICD11: 1B10 | [476] |
Quazepam |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [477] |
Rosuvastatin |
Drug Info | Approved | Hypertriglyceridaemia | ICD11: 5C80 | [478] |
Salmeterol xinafoate |
Drug Info | Approved | Asthma | ICD11: CA23 | [479] |
Secnidazole |
Drug Info | Approved | Amebiasis | ICD11: 1A36 | [480] |
Selegiline |
Drug Info | Approved | Major depressive disorder | ICD11: 6A70-6A7Z | [481] |
Selegiline hydrochloride |
Drug Info | Approved | Parkinsonism | ICD11: 8A00 | [482] |
Selexipag |
Drug Info | Approved | Pulmonary hypertension | ICD11: BB01 | [483] |
Sertraline hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [312] |
Sibutramine |
Drug Info | Approved | Obesity | ICD11: 5B81 | [484] |
Sildenafil citrate |
Drug Info | Approved | Erectile dysfunction | ICD11: HA01 | [24] |
Stiripentol |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [485] |
Sulindac |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [486] |
Tazemetostat |
Drug Info | Approved | Follicular lymphoma | ICD11: 2A80 | [487] |
Temazepam |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [488] |
Tetracycline |
Drug Info | Approved | Spotted fever | ICD11: 1C31 | [489] |
Tiagabine |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [490] |
Tinidazole |
Drug Info | Approved | Protozoan infection | ICD11: 1F5Z | [491] |
Tiotropium |
Drug Info | Approved | Chronic obstructive pulmonary disease | ICD11: CA22 | [492] |
Tiotropium Bromide |
Drug Info | Approved | Chronic obstructive pulmonary disease | ICD11: CA22 | [492] |
Tolvaptan |
Drug Info | Approved | Hyponatraemia | ICD11: 5C72 | [493] |
Triclabendazole |
Drug Info | Approved | Fascioliasis | ICD11: 1F82 | [494] |
Ulipristal |
Drug Info | Approved | Contraception | ICD11: JA65 | [495] |
Vandetanib |
Drug Info | Approved | Thyroid cancer | ICD11: 2D10 | [496] |
Vorapaxar sulfate |
Drug Info | Approved | Myocardial infarction | ICD11: BA41 | [497] |
Voxelotor |
Drug Info | Approved | Sickle-cell anaemia | ICD11: 3A51 | [367] |
Zonisamide |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [498] |
Avacopan |
Drug Info | Approved | Discovery agent | ICD: N.A. | [499] |
Aztreonam |
Drug Info | Approved | Cystic fibrosis | ICD11: CA25 | [500] |
Baloxavir marboxil |
Drug Info | Approved | Influenza virus infection | ICD11: 1E30 | [501] |
Baloxavir marboxil |
Drug Info | Approved | Influenza virus infection | ICD11: 1E30 | [502] |
Bexarotene |
Drug Info | Approved | Anaplastic large cell lymphoma | ICD11: 2A90 | [18] |
Bimatoprost |
Drug Info | Approved | Glaucoma | ICD11: 9C61 | [503] |
Bisoprolol |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [504] |
Bosutinib |
Drug Info | Approved | Chronic myelogenous leukaemia | ICD11: 2A20 | [6] |
Brinzolamide |
Drug Info | Approved | Glaucoma | ICD11: 9C61 | [18] |
Chloroquine |
Drug Info | Approved | Malaria | ICD11: 1F40 | [505] |
Chloroquine |
Drug Info | Approved | Malaria | ICD11: 1F40 | [506] |
Chlorzoxazone |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [18] |
Clevidipine |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [507] |
Clotrimazole |
Drug Info | Approved | Candidiasis | ICD11: 1F23 | [508] |
Conivaptan hydrochloride |
Drug Info | Approved | Hyponatraemia | ICD11: 5C72 | [509] |
Dacomitinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [510] |
Dantrolene sodium |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [18] |
Desipramine |
Drug Info | Approved | Attention deficit hyperactivity disorder | ICD11: 6A05 | [225] |
Dihydroergocristine |
Drug Info | Approved | Alcohol dependence | ICD11: 6C40 | [511] |
Dirithromycin |
Drug Info | Approved | Bronchitis | ICD11: CA20 | [512] |
Disopyramide phosphate |
Drug Info | Approved | Ventricular tachyarrhythmia | ICD11: BC71 | [513] |
Dorzolamide hydrochloride |
Drug Info | Approved | Glaucoma | ICD11: 9C61 | [18] |
Elbasvir |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [514] |
Enasidenib |
Drug Info | Approved | Acute myeloid leukaemia | ICD11: 2A60 | [515] |
Encorafenib |
Drug Info | Approved | Melanoma | ICD11: 2C30 | [516] |
Epinastine |
Drug Info | Approved | Conjunctivitis | ICD11: 9A60 | [517] |
Erythromycin stearate |
Drug Info | Approved | Acute upper respiratory infection | ICD11: CA07 | [518] |
Ethchlorvynol |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [519] |
Felodipine |
Drug Info | Approved | Essential hypertension | ICD11: BA00 | [520] |
Fluprednisolone |
Drug Info | Approved | Asthma | ICD11: CA23 | [521] |
Fluticasone furoate |
Drug Info | Approved | Allergic rhinitis | ICD11: CA08 | [118] |
Fluticasone propionate |
Drug Info | Approved | Asthma | ICD11: CA23 | [522] |
Fluticasone propionate |
Drug Info | Approved | Asthma | ICD11: CA23 | [523] |
Fosinopril |
Drug Info | Approved | Hypertension | ICD11: BA00-BA04 | [524] |
Fulvestrant |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [525] |
GDC-0199 |
Drug Info | Approved | Solid tumour/cancer | ICD11: 2A00-2F9Z | [526] |
GS-7340 |
Drug Info | Approved | Hepatitis B virus infection | ICD11: 1E50-1E51 | [527] |
Halcinonide |
Drug Info | Approved | Atopic dermatitis | ICD11: EA80 | [528] |
Halofantrine |
Drug Info | Approved | Malaria | ICD11: 1F40 | [529] |
Hydroxyamphetamine |
Drug Info | Approved | Horner syndrome | ICD11: 8D8A | [530] |
Hydroxychloroquine sulfate |
Drug Info | Approved | Malaria | ICD11: 1F40 | [531] |
Hydroxyprogesterone caproate |
Drug Info | Approved | Uterine cancer | ICD11: 2C76 | [532] |
Hydroxyzine |
Drug Info | Approved | Anxiety disorder | ICD11: 6B00 | [533] |
Ibudilast |
Drug Info | Approved | Castleman's disease | ICD11: 4B2Y | [534] |
Imiquimod |
Drug Info | Approved | Basal cell carcinoma | ICD11: 2C32 | [18] |
Indacaterol maleate |
Drug Info | Approved | Chronic obstructive pulmonary disease | ICD11: CA22 | [535] |
Isavuconazonium |
Drug Info | Approved | Allergic bronchopulmonary aspergillosis | ICD11: CA82 | [536] |
Lefamulin |
Drug Info | Approved | Pneumocystis pneumonia | ICD11: CA40 | [537] |
Lenacapavir |
Drug Info | Approved | Discovery agent | ICD: N.A. | [538] |
Lenacapavir |
Drug Info | Approved | Discovery agent | ICD: N.A. | [539] |
Letrozole |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [540] |
Lonafarnib |
Drug Info | Approved | Progeria | ICD11: LD2B | [541], [542] |
Lonafarnib |
Drug Info | Approved | Progeria | ICD11: LD2B | [541] |
Lorlatinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [543] |
Lumefantrine |
Drug Info | Approved | Malaria | ICD11: 1F40 | [544] |
Meloxicam |
Drug Info | Approved | Osteoarthritis | ICD11: FA00 | [545] |
Methylergometrine |
Drug Info | Approved | Migraine | ICD11: 8A80 | [442] |
Methysergide |
Drug Info | Approved | Migraine | ICD11: 8A80 | [546] |
Mitapivat |
Drug Info | Approved | Discovery agent | ICD: N.A. | [547] |
Modafinil |
Drug Info | Approved | Narcolepsy | ICD11: 7A20 | [18] |
Nebivolol hydrochloride |
Drug Info | Approved | Pulmonary hypertension | ICD11: BB01 | [548] |
Norgestimate |
Drug Info | Approved | Acne vulgaris | ICD11: ED80 | [549] |
Nortriptyline hydrochloride |
Drug Info | Approved | Depression | ICD11: 6A71 | [550] |
Omidenepag isopropyl |
Drug Info | Approved | Discovery agent | ICD: N.A. | [551] |
Orlistat |
Drug Info | Approved | Obesity | ICD11: 5B80-5B81 | [552] |
Oxybutynin chloride |
Drug Info | Approved | Overactive bladder | ICD11: GC50 | [553] |
Paramethasone |
Drug Info | Approved | Allergy | ICD11: 4A80 | [118] |
Pemigatinib |
Drug Info | Approved | Hepatocellular carcinoma | ICD11: 2C12 | [554] |
Pemigatinib |
Drug Info | Approved | Hepatocellular carcinoma | ICD11: 2C12 | [555] |
Pemigatinib |
Drug Info | Approved | Hepatocellular carcinoma | ICD11: 2C12 | [556] |
Pemigatinib |
Drug Info | Approved | Hepatocellular carcinoma | ICD11: 2C12 | [557] |
Permethrin |
Drug Info | Approved | Scabies | ICD11: 1G04 | [558] |
Pimecrolimus |
Drug Info | Approved | Atopic dermatitis | ICD11: EA80 | [559] |
Posaconazole |
Drug Info | Approved | Candidiasis | ICD11: 1F23 | [560] |
Praziquantel |
Drug Info | Approved | Schistosomiasis | ICD11: 1F86 | [561] |
Prednicarbate |
Drug Info | Approved | Ascariasis | ICD11: 1F62 | [562] |
Rifampicin |
Drug Info | Approved | Osteoporosis | ICD11: FB83 | [563] |
Rifapentine |
Drug Info | Approved | Pulmonary tuberculosis | ICD11: 1B10 | [564] |
Rifaximin |
Drug Info | Approved | Hepatic encephalopathy | ICD11: DB99 | [565] |
Rimegepant |
Drug Info | Approved | Migraine | ICD11: 8A80 | [566] |
Rimegepant |
Drug Info | Approved | Migraine | ICD11: 8A80 | [567] |
Rimexolone |
Drug Info | Approved | Anterior uveitis | ICD11: 9A96 | [568] |
Ripretinib |
Drug Info | Approved | Gastrointestinal stromal tumour | ICD11: 2B5B | [569] |
Risdiplam |
Drug Info | Approved | Muscular atrophy | ICD11: 8B61 | [570] |
Risperidone |
Drug Info | Approved | Schizophrenia | ICD11: 6A20 | [18] |
Roflumilast |
Drug Info | Approved | Chronic obstructive pulmonary disease | ICD11: CA22 | [571] |
Rolapitant hydrochloride |
Drug Info | Approved | Functional nausea/vomiting | ICD11: DD90 | [572] |
Romidepsin |
Drug Info | Approved | Cutaneous T-cell lymphoma | ICD11: 2B01 | [573] |
Saxagliptin hydrochloride |
Drug Info | Approved | Diabetes mellitus | ICD11: 5A10 | [574] |
Selpercatinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [575] |
Selpercatinib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [318], [575], [576] |
Sevoflurane |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [577] |
Simeprevir sodium |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [578] |
Sufentanil |
Drug Info | Approved | Neuropathic pain | ICD11: 8E43 | [579] |
Tadalafil |
Drug Info | Approved | Erectile dysfunction | ICD11: HA01 | [580] |
Tenapanor |
Drug Info | Approved | Hyperphosphatemia | ICD11: 5C64 | [581] |
Tenofovir alafenamide |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [582] |
Tenofovir disoproxil fumarate |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [582] |
Testosterone cypionate |
Drug Info | Approved | Testosterone deficiency | ICD11: 5A81 | [583] |
Tilidine |
Drug Info | Approved | Pain | ICD11: MG30-MG3Z | [584] |
Triamcinolone diacetate |
Drug Info | Approved | Allergy | ICD11: 4A80 | [585] |
Trilostane |
Drug Info | Approved | Cushing syndrome | ICD11: 5A70 | [586] |
Trimethadione |
Drug Info | Approved | Epilepsy | ICD11: 8A60 | [587] |
Troleandomycin |
Drug Info | Approved | Acute upper respiratory infection | ICD11: CA07 | [588] |
Vardenafil hydrochloride |
Drug Info | Approved | Erectile dysfunction | ICD11: HA01 | [589] |
Velpatasvir |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [590] |
Velpatasvir |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [591] |
Venetoclax |
Drug Info | Approved | Chronic lymphocytic leukaemia | ICD11: 2A82 | [526] |
Vinblastine sulfate |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [592] |
Vincristine sulfate |
Drug Info | Approved | Acute lymphoblastic leukemia | ICD11: 2B33 | [593] |
Vitamin D |
Drug Info | Approved | Hyperparathyroidism | ICD11: 5A51 | [594] |
Voxilaprevir |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [595] |
Voxilaprevir |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [596] |
Zalcitabine |
Drug Info | Approved | Human immunodeficiency virus infection | ICD11: 1C60 | [597] |
Alclometasone |
Drug Info | Approved | Atopic dermatitis | ICD11: EA80 | [598] |
Alfuzosin |
Drug Info | Approved | Prostatic hyperplasia | ICD11: GA90 | [599] |
Alfuzosin |
Drug Info | Approved | Prostatic hyperplasia | ICD11: GA90 | [600] |
Amcinonide |
Drug Info | Approved | Allergic contact dermatitis | ICD11: EK00 | [118] |
Atovaquone |
Drug Info | Approved | Pneumocystis pneumonia | ICD11: CA40 | [601] |
Azithromycin |
Drug Info | Approved | Congenital syphilis | ICD11: 1A60 | [18] |
Bepridil hydrochloride |
Drug Info | Approved | Angina pectoris | ICD11: BA40 | [507] |
Cefalexin |
Drug Info | Approved | Acute otitis media | ICD11: AB00 | [602] |
Clobetasol propionate |
Drug Info | Approved | Psoriasis vulgaris | ICD11: EA90 | [603] |
Corticotropin |
Drug Info | Approved | Cushing syndrome | ICD11: 5A70 | [604] |
Desonide |
Drug Info | Approved | Atopic dermatitis | ICD11: EA80 | [18] |
Desoxycorticosterone acetate |
Drug Info | Approved | Osteoporosis | ICD11: FB83 | [605] |
Desoxycorticosterone pivalate |
Drug Info | Approved | Adrenocortical deficiency | ICD11: 5A74 | [605] |
Dexamethasone acetate |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [357] |
Doxycycline |
Drug Info | Approved | Periodontal disease | ICD11: DA0C | [606] |
Ergoloid mesylate |
Drug Info | Approved | Alzheimer disease | ICD11: 8A20 | [607] |
Eribulin |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [608] |
Eribulin mesylate |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [609] |
Flumethasone |
Drug Info | Approved | Atopic dermatitis | ICD11: EA80 | [598] |
Fluocinolone acetonide |
Drug Info | Approved | Atopic dermatitis | ICD11: EA80 | [291] |
Fluocinonide |
Drug Info | Approved | Atopic dermatitis | ICD11: EA80 | [291] |
Flurandrenolide |
Drug Info | Approved | Psoriasis vulgaris | ICD11: EA90 | [598] |
Gemtuzumab ozogamicin |
Drug Info | Approved | Acute myeloid leukaemia | ICD11: 2A60 | [294] |
Glecaprevir |
Drug Info | Approved | Viral hepatitis | ICD11: 1E51 | [610] |
IPI-145 |
Drug Info | Approved | Follicular lymphoma | ICD11: 2A80 | [611] |
Liotrix |
Drug Info | Approved | Hypothyroidism | ICD11: 5A00 | [612] |
Macrolides |
Drug Info | Approved | Bacterial infection | ICD11: 1A00-1C4Z | [613] |
Meprednisone |
Drug Info | Approved | Chronic obstructive pulmonary disease | ICD11: CA22 | [118] |
Methoxsalen |
Drug Info | Approved | Psoriasis vulgaris | ICD11: EA90 | [614] |
Methoxyflurane |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [577] |
Miconazole |
Drug Info | Approved | Dermatophytosis | ICD11: 1F28 | [615] |
Nabilone |
Drug Info | Approved | Insomnia | ICD11: 7A00-7A0Z | [616] |
NERATINIB MALEATE |
Drug Info | Approved | HER2/NEU overexpressing breast cancer | ICD11: 2C60-2C6Y | [617] |
Podophyllotoxin |
Drug Info | Approved | Anogenital warts | ICD11: 1A95 | [618] |
Ramelteon |
Drug Info | Approved | Insomnia | ICD11: 7A00 | [619] |
Retapamulin |
Drug Info | Approved | Impetigo | ICD11: 1B72 | [620] |
Ribociclib succinate |
Drug Info | Approved | Breast cancer | ICD11: 2C60 | [621] |
Testosterone |
Drug Info | Approved | Osteoporosis | ICD11: FB83 | [622] |
Trifarotene |
Drug Info | Approved | Acne vulgaris | ICD11: ED80 | [623] |
Trilaciclib |
Drug Info | Approved | Lung cancer | ICD11: 2C25 | [624] |
Umbralisib |
Drug Info | Approved | Follicular lymphoma | ICD11: 2A80 | [625] |
Upadacitinib |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [626] |
Upadacitinib |
Drug Info | Approved | Rheumatoid arthritis | ICD11: FA20 | [627] |
Voclosporin |
Drug Info | Approved | Lupus erythematosus | ICD11: 4A40 | [628] |
Ziconotide |
Drug Info | Approved | Anaesthesia | ICD11: 8E22 | [507] |
Lestaurtinib |
Drug Info | Approved (orphan drug) | Acute lymphoblastic leukemia | ICD11: 2B33 | [629] |
Berberine |
Drug Info | Phase 4 | Discovery agent | ICD: N.A. | [631] |
Guanfacine |
Drug Info | Phase 4 | Hepatocellular carcinoma | ICD11: 2C12 | [644] |
LAROPIPRANT |
Drug Info | Phase 4 | Atherosclerosis | ICD11: BA80 | [646] |
Neupro |
Drug Info | Phase 4 | Restless legs syndrome | ICD11: 7A80 | [648] |
Ingrezza |
Drug Info | Phase 4 | Dyskinesia | ICD11: 8A02 | [683] |
| Drugs in Phase 4 Clinical Trial | Click to Show/Hide the Full List of Drugs: 88 Drugs | ||||
Etoperidone |
Drug Info | Phase 4 | Depression | ICD11: 6A71 | [630] |
Ethinylestradiol propanesulfonate |
Drug Info | Phase 4 | Prostate cancer | ICD11: 2C82 | [632] |
Desogestrel |
Drug Info | Phase 4 | Female pelvic pain | ICD11: GA34 | [112] |
Mosapride |
Drug Info | Phase 4 | Postoperative ileus | ICD11: DA93 | [633] |
Parecoxib |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [634] |
Reboxetine |
Drug Info | Phase 4 | Depression | ICD11: 6A71 | [635] |
Ebastine |
Drug Info | Phase 4 | Irritable bowel syndrome | ICD11: DD91 | [636] |
Ebastine |
Drug Info | Phase 4 | Irritable bowel syndrome | ICD11: DD91 | [] |
Sertindole |
Drug Info | Phase 4 | Schizophrenia | ICD11: 6A20 | [637] |
Brotizolam |
Drug Info | Phase 4 | Insomnia | ICD11: 7A00 | [638] |
Cyproterone acetate |
Drug Info | Phase 4 | Polycystic ovary syndrome | ICD11: 5A80 | [639] |
Mestranol |
Drug Info | Phase 4 | Menorrhagia | ICD11: GA20 | [640] |
Artemisinin |
Drug Info | Phase 4 | Malaria | ICD11: 1F40 | [641] |
Dapoxetine |
Drug Info | Phase 4 | Ejaculatory dysfunction | ICD11: HA03 | [642] |
Fendiline |
Drug Info | Phase 4 | Atherosclerosis | ICD11: BA80 | [507] |
Barnidipine |
Drug Info | Phase 4 | Essential hypertension | ICD11: BA00 | [643] |
Cinnarizine |
Drug Info | Phase 4 | Haemorrhagic stroke | ICD11: 8B20 | [507] |
Glibenclamide |
Drug Info | Phase 4 | Diabetes mellitus | ICD11: 5A10 | [24] |
Ketobemidone |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [645] |
Mibefradil |
Drug Info | Phase 4 | Essential hypertension | ICD11: BA00 | [647] |
Nimesulide |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [507] |
Piperaquine |
Drug Info | Phase 4 | Malaria | ICD11: 1F40 | [649], [650], [651] |
Bezafibrate |
Drug Info | Phase 4 | Myocardial infarction | ICD11: BA41 | [294] |
Cibenzoline |
Drug Info | Phase 4 | Atrial fibrillation | ICD11: BC81 | [652] |
Methaqualone |
Drug Info | Phase 4 | Insomnia | ICD11: 7A00 | [653] |
Mianserin |
Drug Info | Phase 4 | Depression | ICD11: 6A71 | [303] |
Propiverine |
Drug Info | Phase 4 | Overactive bladder | ICD11: GC50 | [654] |
Roxithromycin |
Drug Info | Phase 4 | Acute lower respiratory infection | ICD11: CA4Z | [655] |
Tropisetron |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [281] |
Vonoprazan |
Drug Info | Phase 4 | Gastro-oesophageal reflux disease | ICD11: DA22 | [656] |
Vonoprazan |
Drug Info | Phase 4 | Gastro-oesophageal reflux disease | ICD11: DA22 | [657] |
Zotepine |
Drug Info | Phase 4 | Schizophrenia | ICD11: 6A20 | [658] |
Astemizole |
Drug Info | Phase 4 | Allergic rhinitis | ICD11: CA08 | [18] |
Bromperidol |
Drug Info | Phase 4 | Schizophrenia | ICD11: 6A20 | [659] |
Dihydrocodeine |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [660] |
Etoricoxib |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [661] |
Fluspirilene |
Drug Info | Phase 4 | Schizophrenia | ICD11: 6A20 | [507] |
Imrecoxib |
Drug Info | Phase 4 | Osteoarthritis | ICD11: FA00 | [662] |
Ipecac |
Drug Info | Phase 4 | Pulmonary tuberculosis | ICD11: 1B10 | [663] |
Lacidipine |
Drug Info | Phase 4 | Essential hypertension | ICD11: BA00 | [664] |
Levamlodipine |
Drug Info | Phase 4 | Essential hypertension | ICD11: BA00 | [33] |
Medifoxamine |
Drug Info | Phase 4 | Depression | ICD11: 6A71 | [18] |
Phenacetin |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [665] |
Terfenadine |
Drug Info | Phase 4 | Allergy | ICD11: 4A80 | [666] |
Tianeptine |
Drug Info | Phase 4 | Depression | ICD11: 6A71 | [667] |
Tranilast |
Drug Info | Phase 4 | Conjunctivitis | ICD11: 9A60 | [507] |
Aminophenazone |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [668] |
Chlorphenamine |
Drug Info | Phase 4 | Allergy | ICD11: 4A80 | [53] |
Clotiazepam |
Drug Info | Phase 4 | Anxiety disorder | ICD11: 6B00 | [669] |
Dextropropoxyphene |
Drug Info | Phase 4 | Lung cancer | ICD11: 2C25 | [670] |
Dihydralazine |
Drug Info | Phase 4 | Essential hypertension | ICD11: BA00 | [666] |
Flunitrazepam |
Drug Info | Phase 4 | Insomnia | ICD11: 7A00 | [671] |
Gestodene |
Drug Info | Phase 4 | Contraception | ICD11: JA65 | [672] |
Gestrinone |
Drug Info | Phase 4 | Endometriosis | ICD11: GA10 | [673] |
Lynestrenol |
Drug Info | Phase 4 | Transsexualism | ICD11: HA60 | [93] |
Perazine |
Drug Info | Phase 4 | Cerebrovascular dementia | ICD11: 6D81 | [674] |
Trimebutine |
Drug Info | Phase 4 | Irritable bowel syndrome | ICD11: DD91 | [507] |
Zopiclone |
Drug Info | Phase 4 | Insomnia | ICD11: 7A00 | [675] |
Azelnidipine |
Drug Info | Phase 4 | Essential hypertension | ICD11: BA00 | [676] |
Benidipine |
Drug Info | Phase 4 | Diabetic nephropathy | ICD11: GB61 | [677] |
Enclomiphene |
Drug Info | Phase 4 | Female infertility | ICD11: GA31 | [678] |
Etizolam |
Drug Info | Phase 4 | Insomnia | ICD11: 7A00 | [679] |
Flucloxacillin |
Drug Info | Phase 4 | Staphylococcus infection | ICD11: 1B73 | [680] |
Grazoprevir |
Drug Info | Phase 4 | Viral hepatitis | ICD11: 1E51 | [681] |
Hydroquinone |
Drug Info | Phase 4 | Melasma | ICD11: ED60 | [682] |
Ketazolam |
Drug Info | Phase 4 | Anxiety disorder | ICD11: 6B00 | [249] |
Mexazolam |
Drug Info | Phase 4 | Anxiety disorder | ICD11: 6B00 | [256] |
Milnacipran |
Drug Info | Phase 4 | Asperger syndrome | ICD11: 6A02 | [684] |
Otilonium |
Drug Info | Phase 4 | Irritable bowel syndrome | ICD11: DD91 | [507] |
Pranlukast |
Drug Info | Phase 4 | Obstructive sleep apnea | ICD11: 7A41 | [685] |
Pregnenolone |
Drug Info | Phase 4 | Schizophrenia | ICD11: 6A20 | [686] |
Proguanil |
Drug Info | Phase 4 | Malaria | ICD11: 1F40 | [687] |
Promegestone |
Drug Info | Phase 4 | Corpus luteum cyst | ICD11: GA18 | [639] |
Rupatadine |
Drug Info | Phase 4 | Allergy | ICD11: 4A80 | [688] |
Beclabuvir |
Drug Info | Phase 4 | Viral hepatitis | ICD11: 1E51 | [689] |
Dienogest |
Drug Info | Phase 4 | Endometriosis | ICD11: GA10 | [639] |
Ergometrine |
Drug Info | Phase 4 | Postpartum haemorrhage | ICD11: JA43 | [690] |
Ergonovine maleate |
Drug Info | Phase 4 | Abnormal vaginal bleeding | ICD11: GA2Y | [690] |
Nomegestrol |
Drug Info | Phase 4 | Hypercoagulability | ICD11: 3B61 | [639] |
Pinaverium |
Drug Info | Phase 4 | Irritable bowel syndrome | ICD11: DD91 | [507] |
Prenylamine |
Drug Info | Phase 4 | Angina pectoris | ICD11: BA40 | [507] |
Tenofovir disoproxil |
Drug Info | Phase 4 | Human immunodeficiency virus infection | ICD11: 1C60 | [582] |
Thiamylal |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [691] |
Ciprofibrate |
Drug Info | Phase 4 | Hypertriglyceridaemia | ICD11: 5C80 | [294] |
Demegestone |
Drug Info | Phase 4 | Corpus luteum cyst | ICD11: GA18 | [692] |
Lidoflazine |
Drug Info | Phase 4 | Cognitive impairment | ICD11: 6D71 | [507] |
Yohimbine |
Drug Info | Phase 4 | Erectile dysfunction | ICD11: HA01 | [693] |
Zomepirac |
Drug Info | Phase 4 | Anaesthesia | ICD11: 8E22 | [694] |
| Drugs in Phase 3 Clinical Trial | Click to Show/Hide the Full List of Drugs: 119 Drugs | ||||
E-3A |
Drug Info | Phase 3 | Turner syndrome | ICD11: LD50 | [695] |
Fruquintinib |
Drug Info | Phase 3 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [696] |
Selumetinib |
Drug Info | Phase 3 | Neurofibromatosis | ICD11: LD2D | [697] |
MK-0859 |
Drug Info | Phase 3 | Atherosclerosis | ICD11: BA80 | [698] |
EPIANDROSTERONE |
Drug Info | Phase 3 | Discovery agent | ICD: N.A. | [699] |
ABL001 |
Drug Info | Phase 3 | Chronic myelogenous leukaemia | ICD11: 2A20 | [700] |
Gemigliptin |
Drug Info | Phase 3 | Diabetic complication | ICD11: 5A2Y | [701] |
Lisuride |
Drug Info | Phase 3 | Parkinsonism | ICD11: 8A00 | [702] |
LY-2484595 |
Drug Info | Phase 3 | Myocardial infarction | ICD11: BA41 | [703] |
PG-490 |
Drug Info | Phase 3 | Diabetes mellitus | ICD11: 5A10 | [704] |
AZD-2171 |
Drug Info | Phase 3 | Lung cancer | ICD11: 2C25 | [705] |
BMS-298585 |
Drug Info | Phase 3 | Diabetes mellitus | ICD11: 5A10 | [706] |
BMS-650032 |
Drug Info | Phase 3 | Viral hepatitis | ICD11: 1E51 | [707] |
GSK2140944 |
Drug Info | Phase 3 | Urinary tract infection | ICD11: GC08 | [708] |
LCZ-696 |
Drug Info | Phase 3 | Myocardial infarction | ICD11: BA41 | [709] |
BAF-312 |
Drug Info | Phase 3 | Multiple sclerosis | ICD11: 8A40 | [72] |
Finerenone |
Drug Info | Phase 3 | Diabetic nephropathy | ICD11: GB61 | [710], [711], [712] |
Finerenone |
Drug Info | Phase 3 | Diabetic nephropathy | ICD11: GB61 | [712] |
Finerenone |
Drug Info | Phase 3 | Diabetic nephropathy | ICD11: GB61 | [713] |
LAS-17177 |
Drug Info | Phase 3 | Functional nausea/vomiting | ICD11: DD90 | [714] |
Omecamtiv mecarbil |
Drug Info | Phase 3 | Heart failure | ICD11: BD10-BD1Z | [715] |
Tivantinib |
Drug Info | Phase 3 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [716] |
ABT-333 |
Drug Info | Phase 3 | Viral hepatitis | ICD11: 1E51 | [717] |
BPI-2009 |
Drug Info | Phase 3 | Lung cancer | ICD11: 2C25 | [718] |
Chlorpromazine |
Drug Info | Phase 3 | Coronavirus Disease 2019 (COVID-19) | ICD11: 1D6Y | [719] |
JTT-705 |
Drug Info | Phase 3 | Acute coronary syndrome | ICD11: BA4Z | [720] |
KW-5338 |
Drug Info | Phase 3 | Gastroparesis | ICD11: DA41 | [721] |
LAU-7b |
Drug Info | Phase 3 | Macular degeneration | ICD11: 9B78 | [722] |
Mavacamten |
Drug Info | Phase 3 | Hypertrophic cardiomyopathy | ICD11: BC43 | [723] |
NE-10064 |
Drug Info | Phase 3 | Cardiac arrhythmia | ICD11: BC65 | [724] |
Nestorone |
Drug Info | Phase 3 | Endometriosis | ICD11: GA10 | [725] |
NSC-122758 |
Drug Info | Phase 3 | Vitamin deficiency | ICD11: 5B55 | [726] |
SCH-417690 |
Drug Info | Phase 3 | Human immunodeficiency virus infection | ICD11: 1C60 | [24] |
TV-5600 |
Drug Info | Phase 3 | Lupus erythematosus | ICD11: 4A40 | [727] |
Alilusem |
Drug Info | Phase 3 | Essential hypertension | ICD11: BA00 | [728] |
Bexagliflozin |
Drug Info | Phase 3 | Type-2 diabetes | ICD11: 5A11 | [729] |
CKD-501 |
Drug Info | Phase 3 | Diabetes mellitus | ICD11: 5A10 | [730] |
ED-71 |
Drug Info | Phase 3 | Osteoporosis | ICD11: FB83 | [731] |
GW-1000 |
Drug Info | Phase 3 | Cerebral vasospasm | ICD11: BA85 | [732] |
Maribavir |
Drug Info | Phase 3 | Cytomegalovirus infection | ICD11: 1D82 | [733] |
MLN4924 |
Drug Info | Phase 3 | Myelodysplastic syndrome | ICD11: 2A37 | [734] |
SB-939 |
Drug Info | Phase 3 | Acute myeloid leukaemia | ICD11: 2A60 | [735] |
Y-100032 |
Drug Info | Phase 3 | Multiple myeloma | ICD11: 2A83 | [736] |
AD-5423 |
Drug Info | Phase 3 | Schizophrenia | ICD11: 6A20 | [737] |
AK-602 |
Drug Info | Phase 3 | Human immunodeficiency virus infection | ICD11: 1C60 | [512] |
Brivanib |
Drug Info | Phase 3 | Breast cancer | ICD11: 2C60-2C6Y | [738] |
HSDB-3466 |
Drug Info | Phase 3 | Psoriasis vulgaris | ICD11: EA90 | [739] |
Losartan |
Drug Info | Phase 3 | Hypertension | ICD11: BA00-BA04 | [740] |
LU-AE58054 |
Drug Info | Phase 3 | Alzheimer disease | ICD11: 8A20 | [741] |
Masitinib |
Drug Info | Phase 3 | Amyotrophic lateral sclerosis | ICD11: 8B60 | [742] |
Pacritinib |
Drug Info | Phase 3 | Chronic myelogenous leukaemia | ICD11: 2A20 | [743] |
Pacritinib |
Drug Info | Phase 3 | Chronic myelogenous leukaemia | ICD11: 2A20 | [744] |
SUVN-G3031 |
Drug Info | Phase 3 | Cognitive impairment | ICD11: 6D71 | [745] |
TAK-875 |
Drug Info | Phase 3 | Diabetes mellitus | ICD11: 5A10 | [746] |
VX-661 |
Drug Info | Phase 3 | Cystic fibrosis | ICD11: CA25 | [747] |
BRN-0077922 |
Drug Info | Phase 3 | Influenza virus infection | ICD11: 1E30 | [748] |
FCU-2711 |
Drug Info | Phase 3 | Diffuse large B-cell lymphoma | ICD11: 2A81 | [749] |
Hydroxychloroquine |
Drug Info | Phase 3 | Malaria | ICD11: 1F40-1F45 | [506] |
KRM-1648 |
Drug Info | Phase 3 | Chlamydial lymphogranuloma | ICD11: 1A80 | [750] |
LEE011 |
Drug Info | Phase 3 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [751] |
LY-450139 |
Drug Info | Phase 3 | Alzheimer disease | ICD11: 8A20 | [752] |
Nitrazepam |
Drug Info | Phase 3 | Insomnia | ICD11: 7A00 | [753] |
R-1124 |
Drug Info | Phase 3 | Functional nausea/vomiting | ICD11: DD90 | [754] |
WSM-3978G |
Drug Info | Phase 3 | Hypertrophic cardiomyopathy | ICD11: BC43 | [755] |
ABC294640 |
Drug Info | Phase 3 | Advanced solid tumour | ICD11: 2A00-2F9Z | [756] |
CR-2017 |
Drug Info | Phase 3 | Irritable bowel syndrome | ICD11: DD91 | [757] |
DW-1030 |
Drug Info | Phase 3 | Cerebral vasospasm | ICD11: BA85 | [758] |
DX-8951 |
Drug Info | Phase 3 | Pancreatic cancer | ICD11: 2C10 | [759] |
Fenfluramine |
Drug Info | Phase 3 | Dravet syndrome | ICD11: 8A61-8A6Z | [760] |
FRC-8653 |
Drug Info | Phase 3 | Essential hypertension | ICD11: BA00 | [761] |
Imatinib |
Drug Info | Phase 3 | Mantle cell lymphoma | ICD11: 2A85 | [762] |
Imatinib |
Drug Info | Phase 3 | Mantle cell lymphoma | ICD11: 2A85 | [763] |
INCB-024360 |
Drug Info | Phase 3 | Renal cell carcinoma | ICD11: 2C90 | [764] |
KW-3902 |
Drug Info | Phase 3 | Congestive heart failure | ICD11: BD10 | [765] |
L-651582 |
Drug Info | Phase 3 | Lung cancer | ICD11: 2C25 | [507] |
NSC-9705 |
Drug Info | Phase 3 | Osteoarthritis | ICD11: FA00 | [766] |
OPC-12759 |
Drug Info | Phase 3 | Peptic ulcer | ICD11: DA61 | [767] |
TD-758 |
Drug Info | Phase 3 | Cerebral vasospasm | ICD11: BA85 | [507] |
BAY-8697661 |
Drug Info | Phase 3 | Hepatocellular carcinoma | ICD11: 2C12 | [768] |
BNP-1350 |
Drug Info | Phase 3 | Ovarian cancer | ICD11: 2C73 | [769] |
CQA 206-291 |
Drug Info | Phase 3 | Parkinsonism | ICD11: 8A00 | [770] |
CV-4093 |
Drug Info | Phase 3 | Essential hypertension | ICD11: BA00 | [771] |
DUP-996 |
Drug Info | Phase 3 | Cognitive impairment | ICD11: 6D71 | [772] |
Ganaxolone |
Drug Info | Phase 3 | Complex partial seizure | ICD11: 8A61-8A6Z | [773] |
JNJ-54135419 |
Drug Info | Phase 3 | Depression | ICD11: 6A71 | [116] |
LAS-X-113 |
Drug Info | Phase 3 | Allergy | ICD11: 4A80 | [636] |
LS-248 |
Drug Info | Phase 3 | Prostate cancer | ICD11: 2C82 | [774] |
LY-317615 |
Drug Info | Phase 3 | Mantle cell lymphoma | ICD11: 2A85 | [775] |
Mirvetuximab soravtansine |
Drug Info | Phase 3 | Ovarian cancer | ICD11: 2C73 | [776] |
MRTX849 |
Drug Info | Phase 3 | Lung cancer | ICD11: 2C25 | [777] |
PR-934423 |
Drug Info | Phase 3 | Anaesthesia | ICD11: 8E22 | [778] |
PT2977 |
Drug Info | Phase 3 | Renal cell carcinoma | ICD11: 2C90 | [779] |
RO-7-0207 |
Drug Info | Phase 3 | Colon cancer | ICD11: 2B90 | [780] |
SHP-620 |
Drug Info | Phase 3 | Cytomegalovirus infection | ICD11: 1D82 | [781] |
Sildenafil |
Drug Info | Phase 3 | Erectile dysfunction | ICD11: HA00-HA01 | [782] |
WY-1485 |
Drug Info | Phase 3 | Haemorrhagic stroke | ICD11: 8B20 | [18] |
ABT-627 |
Drug Info | Phase 3 | Diabetic nephropathy | ICD11: GB61 | [783] |
ABT-761 |
Drug Info | Phase 3 | Asthma | ICD11: CA23 | [784] |
Ag-221 |
Drug Info | Phase 3 | Acute myelogenous leukaemia | ICD11: 2A41 | [785] |
Al3-818 |
Drug Info | Phase 3 | Leiomyosarcoma | ICD11: 2B58 | [786] |
AMD-070 |
Drug Info | Phase 3 | Whim syndrome | ICD11: 4A00 | [787] |
ANA-756 |
Drug Info | Phase 3 | Essential hypertension | ICD11: BA00 | [18] |
ARC-029 |
Drug Info | Phase 3 | Alzheimer disease | ICD11: 8A20 | [788] |
ASP-2151 |
Drug Info | Phase 3 | Herpes simplex virus infection | ICD11: 1F00 | [789] |
Atorvastatin |
Drug Info | Phase 3 | Hypertriglyceridaemia | ICD11: 5C80 | [790] |
CC-25430 |
Drug Info | Phase 3 | Tinnitus | ICD11: AB37 | [507] |
CS-011 |
Drug Info | Phase 3 | Diabetes mellitus | ICD11: 5A10 | [42] |
CYT-387 |
Drug Info | Phase 3 | Myelofibrosis | ICD11: 2A22 | [791] |
DEB-025 |
Drug Info | Phase 3 | Muscular dystrophy | ICD11: 8C70 | [792] |
DRF-2593 |
Drug Info | Phase 3 | Diabetes mellitus | ICD11: 5A10 | [42] |
FT-0755769 |
Drug Info | Phase 3 | Stomach cancer | ICD11: 2B72 | [793] |
GTPL-9088 |
Drug Info | Phase 3 | Atopic dermatitis | ICD11: EA80 | [794] |
HE-3286 |
Drug Info | Phase 3 | Diabetes mellitus | ICD11: 5A10 | [795] |
Montelukast |
Drug Info | Phase 3 | Asthma | ICD11: CA23 | [24] |
Palovarotene |
Drug Info | Phase 3 | Fibrodysplasia ossificans progressiva | ICD11: FB31 | [796] |
RG7388 |
Drug Info | Phase 3 | Acute myeloid leukaemia | ICD11: 2A60 | [797] |
Ridaforolimus |
Drug Info | Phase 3 | Sarcoma | ICD11: 2A60-2C35 | [798] |
SCY-078 |
Drug Info | Phase 3 | Candidiasis | ICD11: 1F23 | [799] |
Solithromycin |
Drug Info | Phase 3 | Bacillus anthracis infection | ICD11: 1G40-1G41 | [800] |
| Drugs in Phase 2 Clinical Trial | Click to Show/Hide the Full List of Drugs: 113 Drugs | ||||
Emixustat |
Drug Info | Phase 2/3 | Age-related macular degeneration | ICD11: 9B75 | [801] |
Verapamil |
Drug Info | Phase 2/3 | Hypertension | ICD11: BA00-BA04 | [802], [803] |
EMD-128130 |
Drug Info | Phase 2/3 | Rett syndrome | ICD11: LD90 | [804] |
Pexacerfont |
Drug Info | Phase 2/3 | Anxiety disorder | ICD11: 6B00-6B0Z | [805] |
Amiodarone |
Drug Info | Phase 2/3 | Ventricular tachyarrhythmia | ICD11: BC71 | [806], [807] |
AY-27,255 |
Drug Info | Phase 2/3 | Haemorrhagic stroke | ICD11: 8B20 | [507] |
DA-8159 |
Drug Info | Phase 2/3 | Pulmonary hypertension | ICD11: BB01 | [808] |
Fexinidazole |
Drug Info | Phase 2/3 | Trypanosomiasis | ICD11: 1D51-1F53 | [809], [810] |
Fexinidazole |
Drug Info | Phase 2/3 | Trypanosomiasis | ICD11: 1D51-1F53 | [810] |
HOE-239 |
Drug Info | Phase 2/3 | Trypanosomiasis | ICD11: 1F51 | [810] |
Lilly-112531 |
Drug Info | Phase 2/3 | Diffuse large B-cell lymphoma | ICD11: 2A81 | [811] |
Ramatroban |
Drug Info | Phase 2/3 | Allergic rhinitis | ICD11: CA08 | [812] |
LDE225 |
Drug Info | Phase 2 | Brain cancer | ICD11: 2A00 | [813] |
PF-00734200 |
Drug Info | Phase 2 | Type-2 diabetes | ICD11: 5A11 | [814] |
UK-453,061 |
Drug Info | Phase 2 | Human immunodeficiency virus infection | ICD11: 1C60 | [815] |
Leniolisib |
Drug Info | Phase 2 | Sjogren syndrome | ICD11: 4A43 | [816] |
ABT-450 |
Drug Info | Phase 2 | Hepatitis C virus infection | ICD11: 1E50-1E51 | [817], [818] |
BRN-0456976 |
Drug Info | Phase 2 | Anaesthesia | ICD11: 8E22 | [819] |
CYC-202 |
Drug Info | Phase 2 | Lung cancer | ICD11: 2C25 | [820] |
NITD609 |
Drug Info | Phase 2 | Malaria | ICD11: 1F40-1F45 | [821] |
Paritaprevir |
Drug Info | Phase 2 | Hepatitis C virus infection | ICD11: 1E50-1E51 | [818] |
PF-06463922 |
Drug Info | Phase 2 | Lung cancer | ICD11: 2C25 | [822] |
BLZ-945 |
Drug Info | Phase 2 | Amyotrophic lateral sclerosis | ICD11: 8B60 | [823] |
BMS-690514 |
Drug Info | Phase 2 | Chronic pain | ICD11: MG30 | [824] |
GS-9620 |
Drug Info | Phase 2 | Viral hepatitis | ICD11: 1E51 | [825] |
LIK-066 |
Drug Info | Phase 2 | Heart failure | ICD11: BD10-BD1Z | [826] |
TD-8954 |
Drug Info | Phase 2 | Gastrointestinal disease | ICD11: DE2Z | [827] |
AZD-2014 |
Drug Info | Phase 2 | Brain cancer | ICD11: 2A00 | [828] |
BAY-1002670 |
Drug Info | Phase 2 | Uterine leiomyoma | ICD11: 2E86 | [829] |
Ethanol |
Drug Info | Phase 2 | Diabetic nephropathy | ICD11: GB61 | [18] |
LJN452 |
Drug Info | Phase 2 | Primary biliary cholangitis | ICD11: DB96 | [830] |
S-297995 |
Drug Info | Phase 2 | Opiate dependence | ICD11: 6C43 | [831] |
(S)-oxybutynin |
Drug Info | Phase 2 | Urinary incontinence | ICD11: MF50 | [832] |
MT-1303 |
Drug Info | Phase 2 | Crohn disease | ICD11: DD70 | [833] |
PT2385 |
Drug Info | Phase 2 | Von hippel-lindau disease | ICD11: 5A75 | [834] |
VATALANIB |
Drug Info | Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [835] |
VATALANIB |
Drug Info | Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [836] |
AP26113 |
Drug Info | Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [837] |
AP26113 |
Drug Info | Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [838] |
BMS-275183 |
Drug Info | Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [839] |
CBL-0102 |
Drug Info | Phase 2 | Creutzfeldt-Jakob disease | ICD11: 8E02 | [840] |
GSK2269557 |
Drug Info | Phase 2 | Asthma | ICD11: CA23 | [841] |
JNJ-53718678 |
Drug Info | Phase 2 | Respiratory syncytial virus infection | ICD11: 1C80 | [842] |
KNI-272 |
Drug Info | Phase 2 | Human immunodeficiency virus infection | ICD11: 1C60 | [843] |
Remogliflozin etabonate |
Drug Info | Phase 2 | Type-1/2 diabetes | ICD11: 5A10-5A11 | [844] |
SSR-97193 |
Drug Info | Phase 2 | Malaria | ICD11: 1F40 | [845] |
BIIB-014 |
Drug Info | Phase 2 | Parkinsonism | ICD11: 8A00 | [846] |
Gepirone |
Drug Info | Phase 2 | Depression | ICD11: 6A70-6A7Z | [847] |
HE-3235 |
Drug Info | Phase 2 | Breast cancer | ICD11: 2C60 | [848] |
HP-184 |
Drug Info | Phase 2 | Multiple sclerosis | ICD11: 8A40 | [849] |
Org-33062 |
Drug Info | Phase 2 | Cocaine addiction | ICD11: 6C45 | [850] |
PF-04991532 |
Drug Info | Phase 2 | Diabetic complication | ICD11: 5A2Y | [851] |
Saracatinib |
Drug Info | Phase 2 | Osteosarcoma | ICD11: 2B51 | [852] |
BMS-911543 |
Drug Info | Phase 2 | Chronic myelogenous leukaemia | ICD11: 2A20 | [853] |
CP-122721 |
Drug Info | Phase 2 | Depression | ICD11: 6A71 | [854] |
EXR-202 |
Drug Info | Phase 2 | Parkinsonism | ICD11: 8A00 | [18] |
Ibrolipim |
Drug Info | Phase 2 | Hypertriglyceridaemia | ICD11: 5C80 | [855] |
PD-115934 |
Drug Info | Phase 2 | Breast cancer | ICD11: 2C60 | [476] |
Vincamine |
Drug Info | Phase 2 | Cerebrovascular dementia | ICD11: 6D81 | [414] |
Ym-758 |
Drug Info | Phase 2 | Angina pectoris | ICD11: BA40 | [856] |
Z-4828 |
Drug Info | Phase 2 | Histiocytic sarcoma | ICD11: 2B31 | [857] |
ABT-384 |
Drug Info | Phase 2 | Viral hepatitis | ICD11: 1E51 | [858] |
AR-67 |
Drug Info | Phase 2 | Brain cancer | ICD11: 2A00 | [859] |
AS-703026 |
Drug Info | Phase 2 | Mantle cell lymphoma | ICD11: 2A85 | [860] |
AZD2624 |
Drug Info | Phase 2 | Multiple sclerosis | ICD11: 8A40 | [861] |
CT-1501R |
Drug Info | Phase 2 | Diabetes mellitus | ICD11: 5A10 | [862] |
D-600 |
Drug Info | Phase 2 | Asthma | ICD11: CA23 | [863] |
EBP-994 |
Drug Info | Phase 2 | Progeria | ICD11: LD2B | [541] |
HPPH |
Drug Info | Phase 2 | Lung cancer | ICD11: 2C25 | [864] |
ICN-2001-3 |
Drug Info | Phase 2 | Viral hepatitis | ICD11: 1E51 | [865] |
JI-101 |
Drug Info | Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [866] |
LY-2603618 |
Drug Info | Phase 2 | Pancreatic cancer | ICD11: 2C10 | [867] |
LY-333531 |
Drug Info | Phase 2 | Breast cancer | ICD11: 2C60 | [868] |
MB-07811 |
Drug Info | Phase 2 | Hypertriglyceridaemia | ICD11: 5C80 | [869] |
MK-3118 |
Drug Info | Phase 2 | Candidiasis | ICD11: 1F23 | [870] |
MK-3118 |
Drug Info | Phase 2 | Candidiasis | ICD11: 1F23 | [871] |
NCI-C50000 |
Drug Info | Phase 2 | Pruritus | ICD11: EC90 | [507] |
PNU-100480 |
Drug Info | Phase 2 | Pulmonary tuberculosis | ICD11: 1B10 | [872] |
SAM-531 |
Drug Info | Phase 2 | Alzheimer disease | ICD11: 8A20 | [873] |
Verubulin |
Drug Info | Phase 2 | Brain cancer | ICD11: 2A00 | [874] |
VX-680 |
Drug Info | Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [875] |
ZK-31224 |
Drug Info | Phase 2 | Pulmonary hypertension | ICD11: BB01 | [876] |
ABT-263 |
Drug Info | Phase 2 | Ovarian cancer | ICD11: 2C73 | [877] |
AZD-2327 |
Drug Info | Phase 2 | Anxiety disorder | ICD11: 6B00-6B0Z | [878] |
BC-3781 |
Drug Info | Phase 2 | Pneumocystis pneumonia | ICD11: CA40 | [879] |
EZN-2208 |
Drug Info | Phase 2 | Colorectal cancer | ICD11: 2B91 | [880] |
GS-5806 |
Drug Info | Phase 2 | Respiratory syncytial virus infection | ICD11: 1C80 | [881] |
MKC-442 |
Drug Info | Phase 2 | Human immunodeficiency virus infection | ICD11: 1C60 | [882] |
NSC-603071 |
Drug Info | Phase 2 | Ovarian cancer | ICD11: 2C73 | [883] |
Nuplazid |
Drug Info | Phase 2 | Alzheimer disease | ICD11: 8A20 | [884] |
Oxfendazole |
Drug Info | Phase 2 | Trichuris trichiura infection | ICD11: 1F6G | [885] |
PF-04937319 |
Drug Info | Phase 2 | Type-2 diabetes | ICD11: 5A11 | [886] |
PTC299 |
Drug Info | Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [887] |
RG-7916 |
Drug Info | Phase 2 | Muscular atrophy | ICD11: 8B61 | [888] |
RO-5190591 |
Drug Info | Phase 2 | Viral hepatitis | ICD11: 1E51 | [889] |
TAK-063 |
Drug Info | Phase 2 | Schizophrenia | ICD11: 6A20 | [890] |
TAK-272 |
Drug Info | Phase 2 | Type-2 diabetes | ICD11: 5A11 | [891] |
TAK-906 |
Drug Info | Phase 2 | Gastroparesis | ICD11: DA41 | [892] |
Tesofensine |
Drug Info | Phase 2 | Pain | ICD11: MG30-MG3Z | [893] |
Tocopherol |
Drug Info | Phase 2 | Phenylketonuria | ICD11: 5C50 | [894] |
CC-220 |
Drug Info | Phase 2 | Sarcoidosis | ICD11: 4B20 | [895] |
Encequidar |
Drug Info | Phase 2 | Metastatic breast cancer | ICD11: 2C60-2C6Y | [896] |
Estetrol |
Drug Info | Phase 2 | Diabetes mellitus | ICD11: 5A10 | [897] |
GS-9857 |
Drug Info | Phase 2 | Hepatitis C virus infection | ICD11: 1E50-1E51 | [898] |
GSK2330672 |
Drug Info | Phase 2 | Pruritus | ICD11: EC90 | [899] |
HY-126236 |
Drug Info | Phase 2 | Congestive heart failure | ICD11: BD10 | [900] |
JNJ-37822681 |
Drug Info | Phase 2 | Schizophrenia | ICD11: 6A20 | [901] |
KD025 |
Drug Info | Phase 2 | Psoriasis vulgaris | ICD11: EA90 | [902] |
KW-4354 |
Drug Info | Phase 2 | Muscular dystrophy | ICD11: 8C70 | [903] |
N-DESMETHYLCLOZAPINE |
Drug Info | Phase 2 | Schizophrenia | ICD11: 6A20 | [904] |
RWJ-241947 |
Drug Info | Phase 2 | Fatty liver disease | ICD11: DB92 | [42] |
SHR-1020 |
Drug Info | Phase 2 | Breast cancer | ICD11: 2C60 | [905] |
TAK-652 |
Drug Info | Phase 2 | Human immunodeficiency virus infection | ICD11: 1C60 | [906] |
| Drugs in Phase 1 Clinical Trial | Click to Show/Hide the Full List of Drugs: 47 Drugs | ||||
GEA-6414 |
Drug Info | Phase 1/2 | Parkinsonism | ICD11: 8A00 | [507] |
CB-3304 |
Drug Info | Phase 1/2 | Chronic lymphocytic leukaemia | ICD11: 2A82 | [907] |
CC-223 |
Drug Info | Phase 1/2 | Lung cancer | ICD11: 2C25 | [908] |
LS-172108 |
Drug Info | Phase 1/2 | Cerebral stroke | ICD11: 8B11 | [909] |
CCRIS-1920 |
Drug Info | Phase 1/2 | Osteoporosis | ICD11: FB83 | [910] |
KB-2796 |
Drug Info | Phase 1/2 | Migraine | ICD11: 8A80 | [507] |
NOVA-63035 |
Drug Info | Phase 1/2 | Choroidal neovascularization | ICD11: 9B76 | [911] |
BRN-5220127 |
Drug Info | Phase 1/2 | Chlamydial lymphogranuloma | ICD11: 1A80 | [912] |
TRV-130 |
Drug Info | Phase 1/2 | Hypothyroidism | ICD11: 5A00 | [913] |
BRN-3122594 |
Drug Info | Phase 1/2 | Haemorrhoid | ICD11: DB60 | [118] |
GTPL7557 |
Drug Info | Phase 1/2 | Schizophrenia | ICD11: 6A20 | [914] |
LY-2940680 |
Drug Info | Phase 1/2 | Breast cancer | ICD11: 2C60 | [915] |
SB-1317 |
Drug Info | Phase 1/2 | Brain cancer | ICD11: 2A00 | [916] |
ZK-299 |
Drug Info | Phase 1/2 | Contraception | ICD11: JA65 | [917] |
KBP-5074 |
Drug Info | Phase 1/2 | Hypertension | ICD11: BA00-BA04 | [918] |
TJN-324 |
Drug Info | Phase 1/2 | Essential hypertension | ICD11: BA00 | [919] |
AG-1549 |
Drug Info | Phase 1 | Human immunodeficiency virus infection | ICD11: 1C60 | [920] |
STX 64 |
Drug Info | Phase 1 | Prostate cancer | ICD11: 2C82 | [921] |
MK-0822 |
Drug Info | Phase 1 | Osteoporosis | ICD11: FB83 | [922] |
AST-1306 |
Drug Info | Phase 1 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [923] |
BE-14348A |
Drug Info | Phase 1 | Viral hepatitis | ICD11: 1E51 | [924] |
H3B-6545 |
Drug Info | Phase 1 | Breast cancer | ICD11: 2C60 | [925] |
M-813 |
Drug Info | Phase 1 | Schizophrenia | ICD11: 6A20 | [926] |
M-813 |
Drug Info | Phase 1 | Schizophrenia | ICD11: 6A20 | [927], [926] |
PPI-2458 |
Drug Info | Phase 1 | Diabetes mellitus | ICD11: 5A10 | [928] |
Alvespimycin hydrochloride |
Drug Info | Phase 1 | Ovarian cancer | ICD11: 2C73 | [929] |
SC-20713 |
Drug Info | Phase 1 | Contraception | ICD11: JA65 | [445] |
Benzoylphenylurea |
Drug Info | Phase 1 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [930], [931] |
DA-1229 |
Drug Info | Phase 1 | Type-2 diabetes | ICD11: 5A11 | [932] |
GS-9883 |
Drug Info | Phase 1 | Human immunodeficiency virus infection | ICD11: 1C60 | [933] |
MP-513 |
Drug Info | Phase 1 | Diabetes mellitus | ICD11: 5A10 | [934] |
SPC-100270 |
Drug Info | Phase 1 | Psoriasis vulgaris | ICD11: EA90 | [935] |
Talinolol |
Drug Info | Phase 1 | Intestine motility disorder | ICD11: DA39 | [936] |
BRN-3224996 |
Drug Info | Phase 1 | Allergy | ICD11: 4A80 | [937] |
CBT-1 |
Drug Info | Phase 1 | Histiocytic sarcoma | ICD11: 2B31 | [507] |
H3B-6527 |
Drug Info | Phase 1 | Hepatocellular carcinoma | ICD11: 2C12 | [938] |
MLN-8054 |
Drug Info | Phase 1 | Colon cancer | ICD11: 2B90 | [939] |
BRN-0498823 |
Drug Info | Phase 1 | Essential hypertension | ICD11: BA00 | [18] |
H3B-8800 |
Drug Info | Phase 1 | Myelodysplastic syndrome | ICD11: 2A37 | [940] |
BI-11634 |
Drug Info | Phase 1 | Thrombosis | ICD11: DB61-GB90 | [941] |
BS-572 |
Drug Info | Phase 1 | Raynaud disease | ICD11: BD42 | [507] |
NSC-675451 |
Drug Info | Phase 1 | Pulmonary tuberculosis | ICD11: 1B10 | [942] |
OSI-930 |
Drug Info | Phase 1 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [943] |
AZD-3839 |
Drug Info | Phase 1 | Alzheimer disease | ICD11: 8A20 | [944] |
G-23350 |
Drug Info | Phase 1 | Venous thromboembolism | ICD11: BD72 | [945] |
ITX-5061 |
Drug Info | Phase 1 | Human immunodeficiency virus infection | ICD11: 1C60 | [946] |
MK-6186 |
Drug Info | Phase 1 | Human immunodeficiency virus infection | ICD11: 1C60-1C62 | [947] |
| Discontinued/withdrawn Drugs | Click to Show/Hide the Full List of Drugs: 49 Drugs | ||||
Ecabapide |
Drug Info | Discontinued in Preregistration | Peptic ulcer | ICD11: DA61 | [948] |
Cilomilast |
Drug Info | Discontinued in Phase 3 | Emphysema | ICD11: CA21 | [949] |
Motesanib |
Drug Info | Discontinued in Phase 3 | Lung cancer | ICD11: 2C25 | [950] |
Darapladib |
Drug Info | Discontinued in Phase 3 | Arteriosclerosis obliterans | ICD11: BD40 | [951] |
Taranabant |
Drug Info | Discontinued in Phase 3 | Obesity | ICD11: 5B80-5B81 | [952] |
Avitriptan |
Drug Info | Discontinued in Phase 3 | Migraine | ICD11: 8A80 | [953] |
BMS-204352 |
Drug Info | Discontinued in Phase 3 | Nerve injury | ICD11: ND56 | [954] |
KRP-297 |
Drug Info | Discontinued in Phase 2 | Hypertriglyceridaemia | ICD11: 5C80 | [955] |
Napitane mesilate |
Drug Info | Discontinued in Phase 2 | Major depressive disorder | ICD11: 6A70-6A7Z | [956] |
Lexacalcitol |
Drug Info | Discontinued in Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [957], [958] |
CP-195543 |
Drug Info | Discontinued in Phase 2 | Rheumatoid arthritis | ICD11: FA20 | [959] |
AZD0328 |
Drug Info | Discontinued in Phase 2 | Alzheimer disease | ICD11: 8A20 | [960] |
Perillyl alcohol |
Drug Info | Discontinued in Phase 2 | Solid tumour/cancer | ICD11: 2A00-2F9Z | [961] |
TAK-802 |
Drug Info | Discontinued in Phase 2 | Urinary dysfunction | ICD11: GC2Z | [962] |
Tetramethylpyrazine |
Drug Info | Discontinued in Phase 2 | Neurological disorder | ICD11: 6B60 | [963] |
YM-992 |
Drug Info | Discontinued in Phase 2 | Depression | ICD11: 6A70-6A7Z | [964] |
BMS-181168 |
Drug Info | Discontinued in Phase 2 | Cognitive impairment | ICD11: 6D71 | [965] |
SINOMENINE |
Drug Info | Discontinued in Phase 2 | Rheumatoid arthritis | ICD11: FA20 | [966] |
AZD0837 |
Drug Info | Discontinued in Phase 2 | Atrial fibrillation | ICD11: BC81 | [967] |
UK-369003 |
Drug Info | Discontinued in Phase 1/2 | Erectile dysfunction | ICD11: HA00-HA01 | [968] |
ABT-107 |
Drug Info | Discontinued in Phase 1 | Attention deficit hyperactivity disorder | ICD11: 6A05 | [969] |
E2101 |
Drug Info | Discontinued in Phase 1 | Dyskinesia | ICD11: 8A02 | [970] |
YM-17E |
Drug Info | Discontinued in Phase 1 | Hypertriglyceridaemia | ICD11: 5C80 | [971] |
JTK-853 |
Drug Info | Discontinued in Phase 1 | Hepatitis C virus infection | ICD11: 1E50-1E51 | [972] |
DPC-681 |
Drug Info | Discontinued in Phase 1 | Human immunodeficiency virus infection | ICD11: 1C60-1C62 | [973] |
CJ-11974 |
Drug Info | Discontinued | Irritable bowel syndrome | ICD11: DD91 | [974] |
CP-529414 |
Drug Info | Discontinued | Hypertriglyceridaemia | ICD11: 5C80 | [975] |
FCF-89 |
Drug Info | Discontinued | Rheumatoid arthritis | ICD11: FA20 | [976] |
CAM-2028 |
Drug Info | Discontinued | Acute nasopharyngitis | ICD11: CA00 | [977] |
Nomifensine |
Drug Info | Discontinued | Depression | ICD11: 6A71 | [978] |
ADD-3878 |
Drug Info | Discontinued | Endometriosis | ICD11: GA10 | [42] |
ML-3000 |
Drug Info | Discontinued | Osteoarthritis | ICD11: FA00 | [24] |
ABT-001 |
Drug Info | Discontinued | Asthma | ICD11: CA23 | [979] |
BMY-42569 |
Drug Info | Discontinued | Depression | ICD11: 6A71 | [980] |
EPO-906 |
Drug Info | Discontinued | Breast cancer | ICD11: 2C60 | [300] |
GTPL-463 |
Drug Info | Discontinued | Lung cancer | ICD11: 2C25 | [507] |
Norcisapride |
Drug Info | Discontinued | Gastro-oesophageal reflux disease | ICD11: DA22 | [981] |
RO-408757 |
Drug Info | Discontinued | Breast cancer | ICD11: 2C60 | [982] |
AN-16649 |
Drug Info | Discontinued | Acute lymphoblastic leukemia | ICD11: 2B33 | [983] |
BMS-488043 |
Drug Info | Discontinued | Human immunodeficiency virus infection | ICD11: 1C60 | [984] |
CB-44212 |
Drug Info | Discontinued | Epilepsy | ICD11: 8A60 | [507] |
GBR-12909 |
Drug Info | Discontinued | Cocaine addiction | ICD11: 6C45 | [985] |
GW-873140 |
Drug Info | Discontinued | Human immunodeficiency virus infection | ICD11: 1C60 | [986] |
ATL-2502 |
Drug Info | Discontinued | Inflammatory bowel disease | ICD11: DD72 | [987] |
GW-766994 |
Drug Info | Discontinued | Asthma | ICD11: CA23 | [988] |
NSC-303812 |
Drug Info | Discontinued | Hyperphosphatemia | ICD11: 5C64 | [989] |
Octopamine |
Drug Info | Discontinued | Thrombosis | ICD11: DB61 | [990] |
RS-8359 |
Drug Info | Discontinued | Depression | ICD11: 6A71 | [991] |
YMB-1002 |
Drug Info | Discontinued | Breast cancer | ICD11: 2C60 | [992] |
| Preclinical/investigative Agents | Click to Show/Hide the Full List of Drugs: 46 Drugs | ||||
EPX-100 |
Drug Info | Preclinical | Dravet syndrome | ICD11: 8A61 | [993] |
VA-10872 |
Drug Info | Preclinical | Anaesthesia | ICD11: 8E22 | [176] |
Q-100155 |
Drug Info | Preclinical | Rheumatoid arthritis | ICD11: FA20 | [663] |
VUFB-11502 |
Drug Info | Preclinical | Amebiasis | ICD11: 1A36 | [994] |
BRN-3148038 |
Drug Info | Preclinical | Schizophrenia | ICD11: 6A20 | [995] |
WIN 55212-2 |
Drug Info | Investigative | Anaesthesia | ICD11: 8E22 | [507] |
Agmatine |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [507] |
Coumarin |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [996] |
Ethylmorphine |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [997] |
Benzotetronic acid |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [998] |
Androstenedione |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [242] |
Butyrfentanyl |
Drug Info | Investigative | Anaesthesia | ICD11: 8E22 | [999] |
Adinazolam |
Drug Info | Investigative | Anxiety disorder | ICD11: 6B00 | [1000] |
Bencyclane |
Drug Info | Investigative | Cerebral vasospasm | ICD11: BA85 | [507] |
Medazepam |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [1001] |
Antipyrine |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [24] |
Clorazepic acid |
Drug Info | Investigative | Anxiety disorder | ICD11: 6B00 | [1002] |
Desmethylastemizole |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [1003] |
Estrone sulfate |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [444] |
Imipramine oxide |
Drug Info | Investigative | Depression | ICD11: 6A71 | [1004] |
Lauric acid |
Drug Info | Investigative | Alzheimer disease | ICD11: 8A20 | [1005] |
Cyamemazine |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [1006] |
Dihydrostreptomycin |
Drug Info | Investigative | Pulmonary tuberculosis | ICD11: 1B10 | [604] |
Equilenin |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [1007] |
Manidipine 2HCl |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [771] |
Niguldipine |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [507] |
Paraoxon |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [1008] |
Chenyltaurine |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [1009] |
CTK1G9578 |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [1010] |
Darodipine |
Drug Info | Investigative | Cerebral ischaemia | ICD11: 8B1Z | [507] |
Dexverapamil |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [507] |
Dotarizine |
Drug Info | Investigative | Migraine | ICD11: 8A80 | [507] |
Ethyl biscoumacetate |
Drug Info | Investigative | Thrombosis | ICD11: DB61 | [1011] |
Flosequinan |
Drug Info | Investigative | Congestive heart failure | ICD11: BD10 | [1012] |
Lorpiprazole |
Drug Info | Investigative | Anxiety disorder | ICD11: 6B00 | [1013] |
Medrogestone |
Drug Info | Investigative | Corpus luteum cyst | ICD11: GA18 | [1014] |
Miocamycin |
Drug Info | Investigative | Pulmonary tuberculosis | ICD11: 1B10 | [1015] |
Nordazepam |
Drug Info | Investigative | Anxiety disorder | ICD11: 6B00 | [488] |
O-Benzylguanine |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [1016] |
Benzodiazepine |
Drug Info | Investigative | Anxiety disorder | ICD11: 6B00 | [18] |
Benzyloxyresorufin |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [1017] |
Chlormadinone |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [692] |
Clorindione |
Drug Info | Investigative | Hypertriglyceridaemia | ICD11: 5C80 | [1018] |
Emopamil |
Drug Info | Investigative | Cerebral ischaemia | ICD11: 8B1Z | [507] |
Erythromycin salnacedin |
Drug Info | Investigative | Discovery agent | ICD: N.A. | [518] |
Niludipine |
Drug Info | Investigative | Cerebral vasospasm | ICD11: BA85 | [507] |
| Tissue/Disease-Specific Protein Abundances of This DME | |||||
|---|---|---|---|---|---|
| Tissue-specific Protein Abundances in Healthy Individuals | Click to Show/Hide | ||||
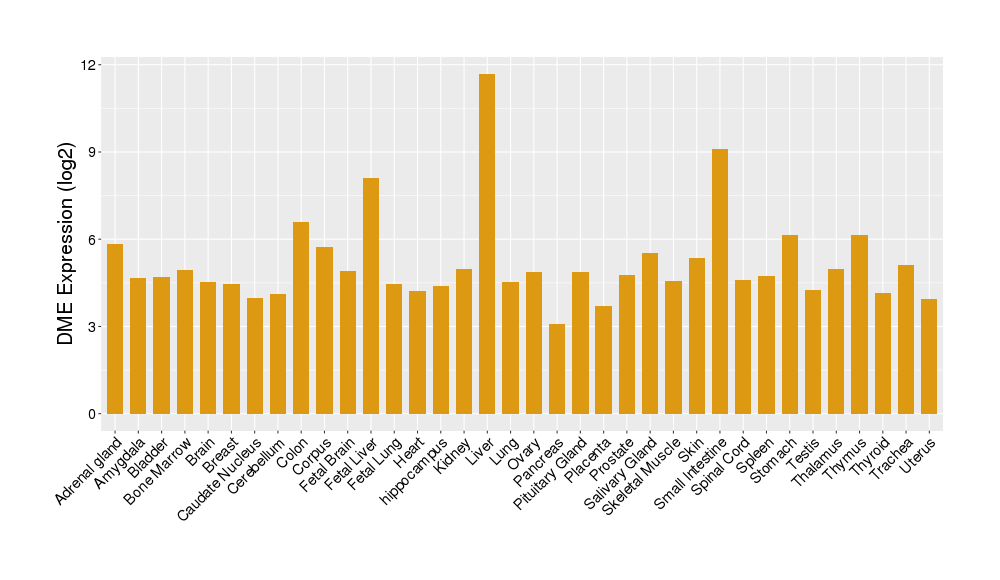
|
|||||
| ICD Disease Classification 01 | Infectious/parasitic disease | Click to Show/Hide | |||
| ICD-11: 1C1H | Necrotising ulcerative gingivitis | Click to Show/Hide | |||
| The Studied Tissue | Gingival tissue | ||||
| The Specified Disease | Bacterial infection of gingival [ICD-11:1C1H] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.79E-02; Fold-change: -8.65E-02; Z-score: -3.88E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 1E30 | Influenza | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Influenza [ICD-11:1E30] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.14E-01; Fold-change: 1.43E-01; Z-score: 6.67E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 1E51 | Chronic viral hepatitis | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Chronic hepatitis C [ICD-11:1E51.1] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.61E-01; Fold-change: 9.46E-03; Z-score: 3.66E-02 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 1G41 | Sepsis with septic shock | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Sepsis with septic shock [ICD-11:1G41] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.10E-04; Fold-change: 6.44E-02; Z-score: 2.85E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
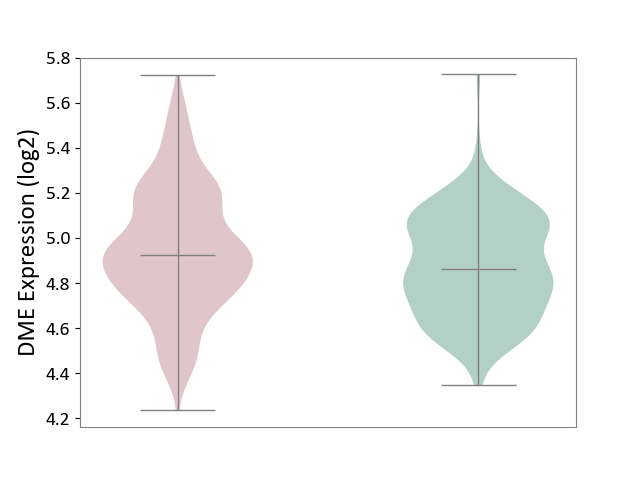
|
Click to View the Clearer Original Diagram | |||
| ICD-11: CA40 | Respiratory syncytial virus infection | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Pediatric respiratory syncytial virus infection [ICD-11:CA40.11] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.52E-01; Fold-change: -1.33E-01; Z-score: -5.91E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
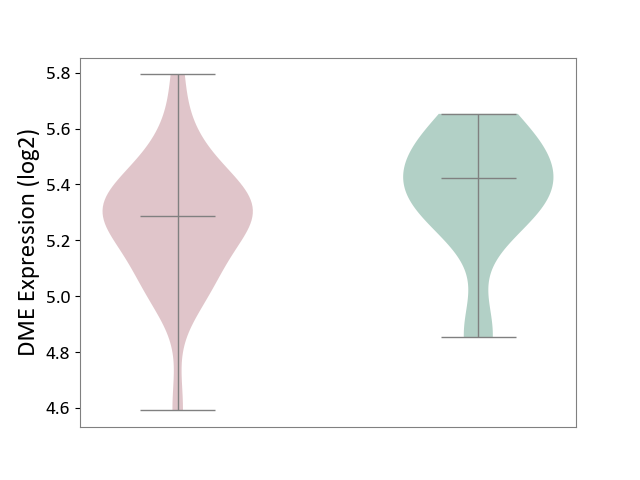
|
Click to View the Clearer Original Diagram | |||
| ICD-11: CA42 | Rhinovirus infection | Click to Show/Hide | |||
| The Studied Tissue | Nasal epithelium tissue | ||||
| The Specified Disease | Rhinovirus infection [ICD-11:CA42.1] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.32E-01; Fold-change: -1.56E-02; Z-score: -1.09E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
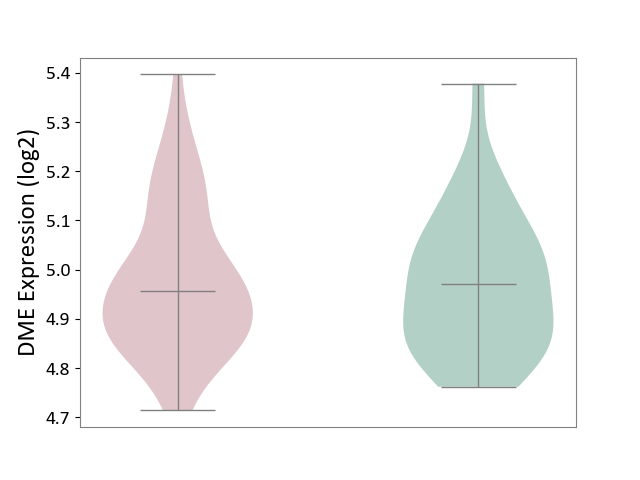
|
Click to View the Clearer Original Diagram | |||
| ICD-11: KA60 | Neonatal sepsis | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Neonatal sepsis [ICD-11:KA60] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.57E-01; Fold-change: -3.39E-02; Z-score: -1.45E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
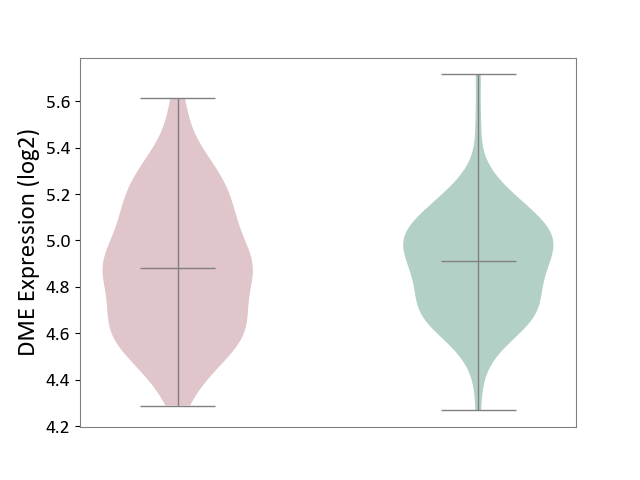
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 02 | Neoplasm | Click to Show/Hide | |||
| ICD-11: 2A00 | Brain cancer | Click to Show/Hide | |||
| The Studied Tissue | Nervous tissue | ||||
| The Specified Disease | Glioblastopma [ICD-11:2A00.00] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.54E-05; Fold-change: -6.60E-02; Z-score: -1.90E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
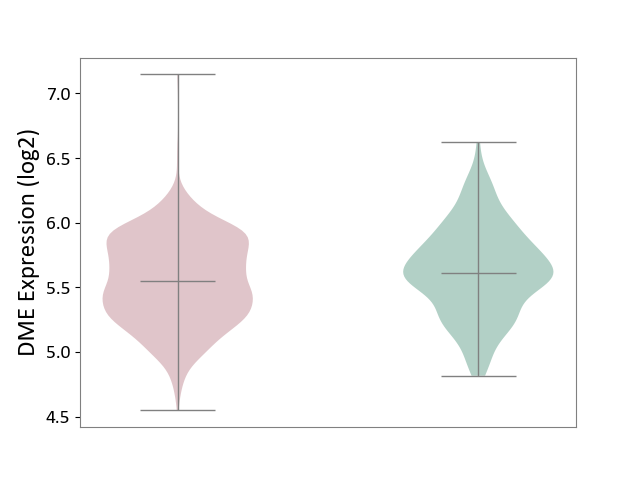
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Brain stem tissue | ||||
| The Specified Disease | Glioma [ICD-11:2A00.0Y-2A00.0Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.50E-01; Fold-change: -9.32E-02; Z-score: -1.01E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
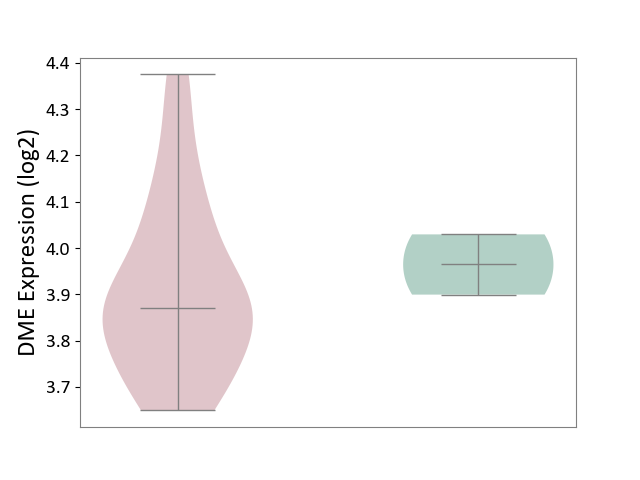
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | White matter tissue | ||||
| The Specified Disease | Glioma [ICD-11:2A00.0Y-2A00.0Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.15E-08; Fold-change: -6.60E-01; Z-score: -3.96E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
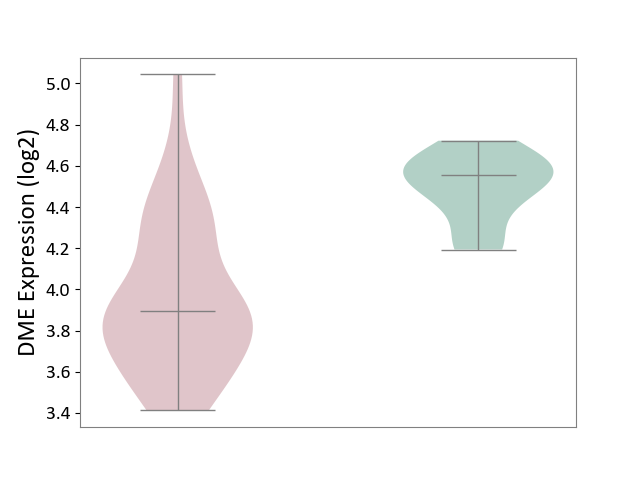
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Brain stem tissue | ||||
| The Specified Disease | Neuroectodermal tumour [ICD-11:2A00.11] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.34E-04; Fold-change: -6.64E-01; Z-score: -1.63E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
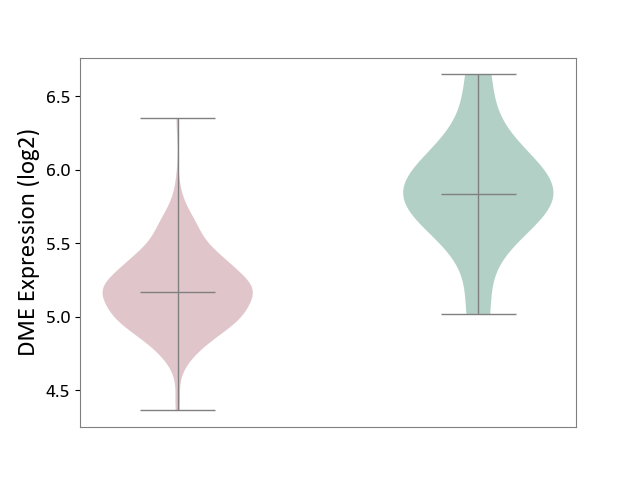
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2A20 | Myeloproliferative neoplasm | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Myelofibrosis [ICD-11:2A20.2] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.97E-01; Fold-change: -3.21E-02; Z-score: -2.80E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
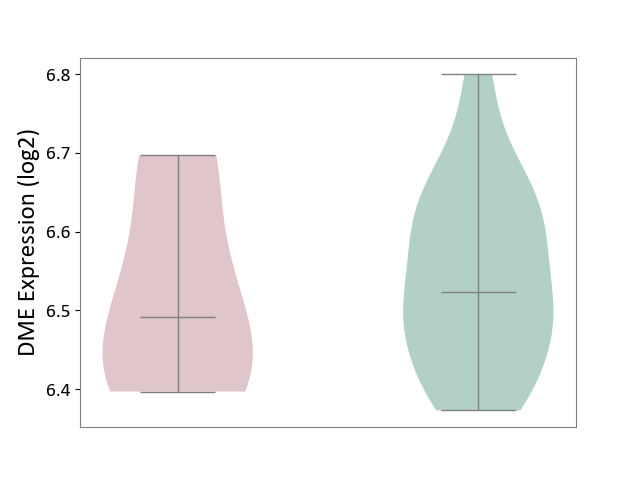
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Polycythemia vera [ICD-11:2A20.4] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.87E-01; Fold-change: -7.38E-02; Z-score: -6.14E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
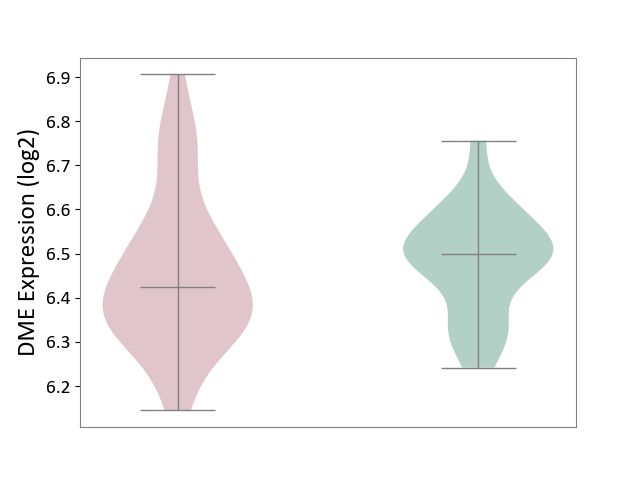
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2A36 | Myelodysplastic syndrome | Click to Show/Hide | |||
| The Studied Tissue | Bone marrow | ||||
| The Specified Disease | Myelodysplastic syndrome [ICD-11:2A36-2A3Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.73E-01; Fold-change: -1.99E-02; Z-score: -1.18E-01 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 6.96E-02; Fold-change: -1.22E-01; Z-score: -1.41E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
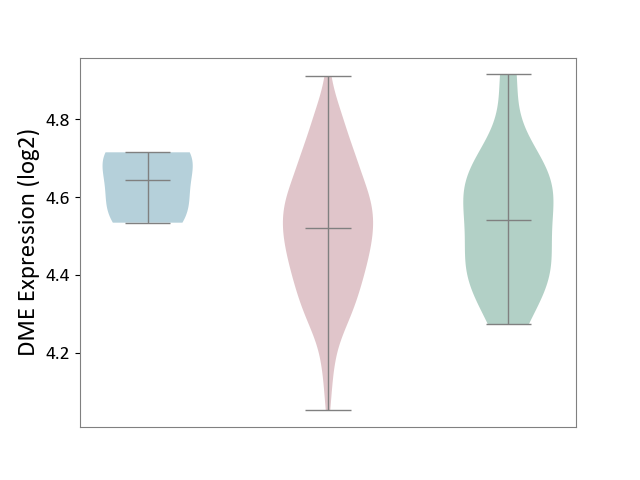
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2A81 | Diffuse large B-cell lymphoma | Click to Show/Hide | |||
| The Studied Tissue | Tonsil tissue | ||||
| The Specified Disease | Diffuse large B-cell lymphoma [ICD-11:2A81] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.00E-01; Fold-change: 4.53E-02; Z-score: 1.94E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
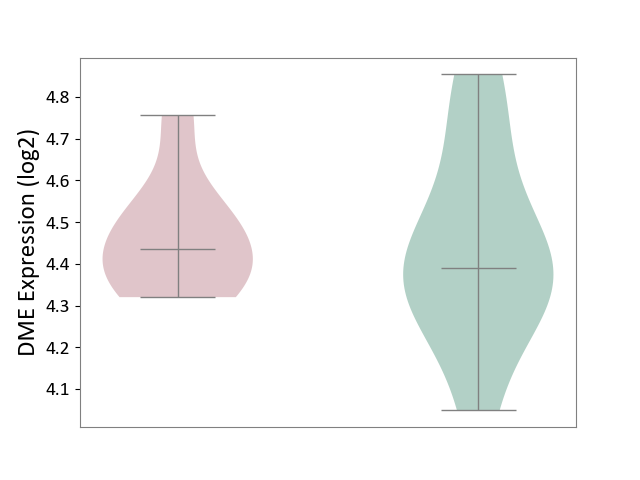
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2A83 | Plasma cell neoplasm | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Multiple myeloma [ICD-11:2A83.1] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.51E-01; Fold-change: 8.39E-02; Z-score: 5.00E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Bone marrow | ||||
| The Specified Disease | Multiple myeloma [ICD-11:2A83.1] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.93E-04; Fold-change: -6.16E-01; Z-score: -2.64E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2B33 | Leukaemia | Click to Show/Hide | |||
| The Studied Tissue | Bone marrow | ||||
| The Specified Disease | Acute myelocytic leukaemia [ICD-11:2B33.1] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.29E-03; Fold-change: -3.47E-02; Z-score: -1.80E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2B6E | Oral cancer | Click to Show/Hide | |||
| The Studied Tissue | Oral tissue | ||||
| The Specified Disease | Oral cancer [ICD-11:2B6E] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.71E-03; Fold-change: -3.53E-01; Z-score: -9.10E-01 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.44E-04; Fold-change: -2.53E-01; Z-score: -6.49E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2B70 | Esophageal cancer | Click to Show/Hide | |||
| The Studied Tissue | Esophagus | ||||
| The Specified Disease | Esophagal cancer [ICD-11:2B70] | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.05E-01; Fold-change: 1.24E-01; Z-score: 7.63E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2B72 | Stomach cancer | Click to Show/Hide | |||
| The Studied Tissue | Gastric tissue | ||||
| The Specified Disease | Gastric cancer [ICD-11:2B72] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.97E-02; Fold-change: 1.84E-01; Z-score: 1.84E+00 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.64E-01; Fold-change: -9.02E-02; Z-score: -1.64E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2B90 | Colon cancer | Click to Show/Hide | |||
| The Studied Tissue | Colon tissue | ||||
| The Specified Disease | Colon cancer [ICD-11:2B90] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.19E-03; Fold-change: -7.56E-02; Z-score: -1.51E-01 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.70E-03; Fold-change: -1.08E-01; Z-score: -2.16E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2B92 | Rectal cancer | Click to Show/Hide | |||
| The Studied Tissue | Rectal colon tissue | ||||
| The Specified Disease | Rectal cancer [ICD-11:2B92] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.90E-02; Fold-change: -3.45E-01; Z-score: -1.49E+00 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.10E-01; Fold-change: -3.09E-01; Z-score: -9.49E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C10 | Pancreatic cancer | Click to Show/Hide | |||
| The Studied Tissue | Pancreas | ||||
| The Specified Disease | Pancreatic cancer [ICD-11:2C10] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.64E-01; Fold-change: -2.20E-01; Z-score: -5.34E-01 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.14E-01; Fold-change: -1.16E-02; Z-score: -8.86E-03 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C12 | Liver cancer | Click to Show/Hide | |||
| The Studied Tissue | Liver tissue | ||||
| The Specified Disease | Liver cancer [ICD-11:2C12.0] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.02E-14; Fold-change: -3.12E+00; Z-score: -2.25E+00 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.09E-84; Fold-change: -2.99E+00; Z-score: -4.75E+00 | ||||
| The Expression Level of Disease Section Compare with the Other Disease Section | p-value: 1.26E-05; Fold-change: -3.27E+00; Z-score: -8.99E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
DME expression in tissue other than the diseased tissue of patients
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C25 | Lung cancer | Click to Show/Hide | |||
| The Studied Tissue | Lung tissue | ||||
| The Specified Disease | Lung cancer [ICD-11:2C25] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.59E-39; Fold-change: -3.76E-01; Z-score: -1.64E+00 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 2.27E-30; Fold-change: -4.60E-01; Z-score: -1.81E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C30 | Skin cancer | Click to Show/Hide | |||
| The Studied Tissue | Skin | ||||
| The Specified Disease | Melanoma [ICD-11:2C30] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.19E-08; Fold-change: -5.72E-01; Z-score: -1.61E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Skin | ||||
| The Specified Disease | Skin cancer [ICD-11:2C30-2C3Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.29E-36; Fold-change: -3.75E-01; Z-score: -1.06E+00 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 5.74E-55; Fold-change: -5.47E-01; Z-score: -1.53E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C6Z | Breast cancer | Click to Show/Hide | |||
| The Studied Tissue | Breast tissue | ||||
| The Specified Disease | Breast cancer [ICD-11:2C60-2C6Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.87E-03; Fold-change: -6.67E-02; Z-score: -2.12E-01 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 5.49E-04; Fold-change: -1.25E-01; Z-score: -5.25E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C73 | Ovarian cancer | Click to Show/Hide | |||
| The Studied Tissue | Ovarian tissue | ||||
| The Specified Disease | Ovarian cancer [ICD-11:2C73] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.96E-01; Fold-change: -3.35E-01; Z-score: -1.10E+00 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 7.51E-01; Fold-change: -7.20E-02; Z-score: -3.51E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C77 | Cervical cancer | Click to Show/Hide | |||
| The Studied Tissue | Cervical tissue | ||||
| The Specified Disease | Cervical cancer [ICD-11:2C77] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.13E-04; Fold-change: -2.15E-01; Z-score: -9.34E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C78 | Uterine cancer | Click to Show/Hide | |||
| The Studied Tissue | Endometrium tissue | ||||
| The Specified Disease | Uterine cancer [ICD-11:2C78] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.20E-02; Fold-change: -1.08E-01; Z-score: -2.31E-01 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.60E-01; Fold-change: 1.36E-01; Z-score: 6.55E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C82 | Prostate cancer | Click to Show/Hide | |||
| The Studied Tissue | Prostate | ||||
| The Specified Disease | Prostate cancer [ICD-11:2C82] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.10E-01; Fold-change: -1.23E-01; Z-score: -2.88E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C90 | Renal cancer | Click to Show/Hide | |||
| The Studied Tissue | Kidney | ||||
| The Specified Disease | Renal cancer [ICD-11:2C90-2C91] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.84E-01; Fold-change: -1.61E-01; Z-score: -3.61E-01 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 2.41E-16; Fold-change: -3.78E-01; Z-score: -1.06E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C92 | Ureter cancer | Click to Show/Hide | |||
| The Studied Tissue | Urothelium | ||||
| The Specified Disease | Ureter cancer [ICD-11:2C92] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.69E-01; Fold-change: 2.43E-02; Z-score: 7.51E-02 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2C94 | Bladder cancer | Click to Show/Hide | |||
| The Studied Tissue | Bladder tissue | ||||
| The Specified Disease | Bladder cancer [ICD-11:2C94] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.23E-11; Fold-change: 4.66E-01; Z-score: 5.83E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
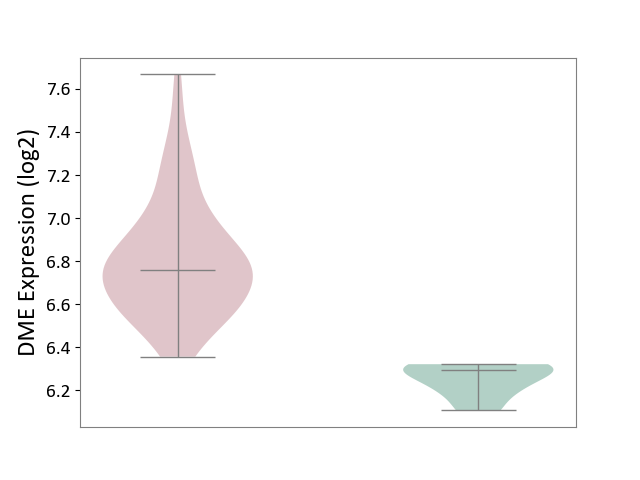
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2D02 | Retinal cancer | Click to Show/Hide | |||
| The Studied Tissue | Uvea | ||||
| The Specified Disease | Retinoblastoma [ICD-11:2D02.2] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.95E-02; Fold-change: -1.55E-01; Z-score: -1.24E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
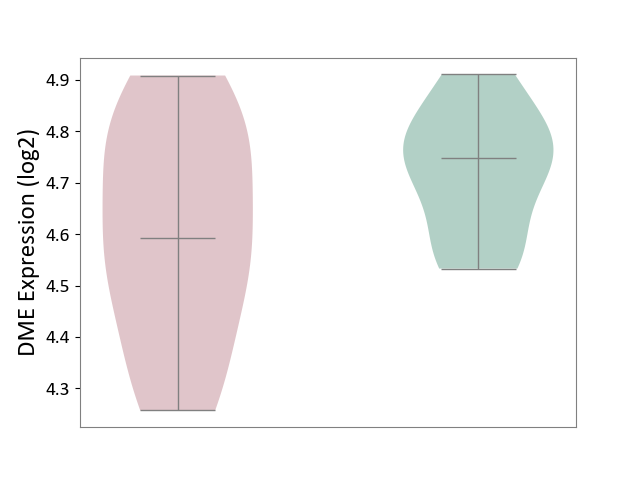
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2D10 | Thyroid cancer | Click to Show/Hide | |||
| The Studied Tissue | Thyroid | ||||
| The Specified Disease | Thyroid cancer [ICD-11:2D10] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.75E-01; Fold-change: 3.78E-02; Z-score: 1.44E-01 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 9.90E-01; Fold-change: 1.53E-02; Z-score: 7.12E-02 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
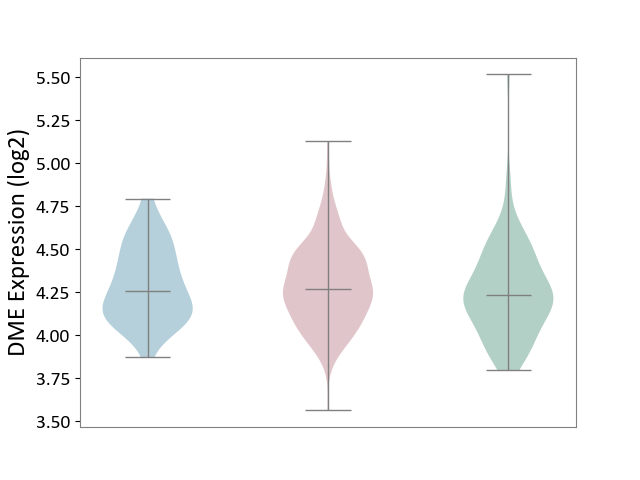
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2D11 | Adrenal cancer | Click to Show/Hide | |||
| The Studied Tissue | Adrenal cortex | ||||
| The Specified Disease | Adrenocortical carcinoma [ICD-11:2D11.Z] | ||||
| The Expression Level of Disease Section Compare with the Other Disease Section | p-value: 1.76E-02; Fold-change: -2.70E-01; Z-score: -4.60E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in tissue other than the diseased tissue of patients
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
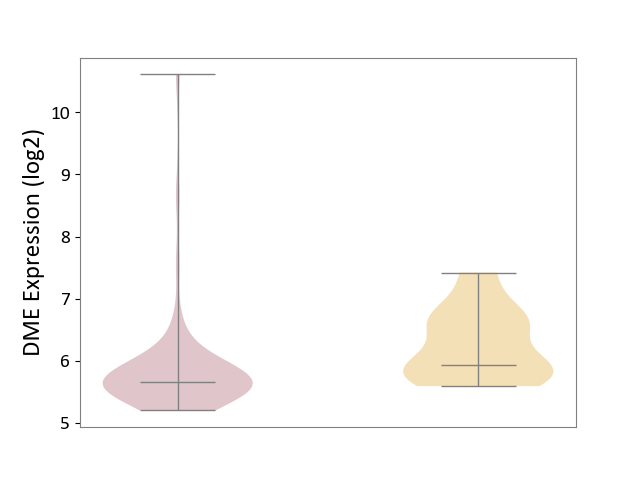
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2D12 | Endocrine gland neoplasm | Click to Show/Hide | |||
| The Studied Tissue | Pituitary tissue | ||||
| The Specified Disease | Pituitary cancer [ICD-11:2D12] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.87E-01; Fold-change: -2.28E-01; Z-score: -4.52E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Pituitary tissue | ||||
| The Specified Disease | Pituitary gonadotrope tumour [ICD-11:2D12] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.30E-01; Fold-change: 2.02E-01; Z-score: 5.47E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 2D42 | Head and neck cancer | Click to Show/Hide | |||
| The Studied Tissue | Head and neck tissue | ||||
| The Specified Disease | Head and neck cancer [ICD-11:2D42] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.04E-04; Fold-change: -9.90E-02; Z-score: -3.91E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 03 | Blood/blood-forming organ disease | Click to Show/Hide | |||
| ICD-11: 3A51 | Sickle cell disorder | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Sickle cell disease [ICD-11:3A51.0-3A51.3] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.21E-01; Fold-change: 1.33E-01; Z-score: 8.26E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 3A70 | Aplastic anaemia | Click to Show/Hide | |||
| The Studied Tissue | Bone marrow | ||||
| The Specified Disease | Shwachman-Diamond syndrome [ICD-11:3A70.0] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.23E-01; Fold-change: 4.92E-01; Z-score: 1.13E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 3B63 | Thrombocytosis | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Thrombocythemia [ICD-11:3B63] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.50E-03; Fold-change: -5.35E-02; Z-score: -4.77E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 3B64 | Thrombocytopenia | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Thrombocytopenia [ICD-11:3B64] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.21E-01; Fold-change: -1.09E-01; Z-score: -6.81E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 04 | Immune system disease | Click to Show/Hide | |||
| ICD-11: 4A00 | Immunodeficiency | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Immunodeficiency [ICD-11:4A00-4A20] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.15E-01; Fold-change: -1.31E-02; Z-score: -9.45E-02 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 4A40 | Lupus erythematosus | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Lupus erythematosus [ICD-11:4A40] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.35E-05; Fold-change: -1.56E-01; Z-score: -4.11E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
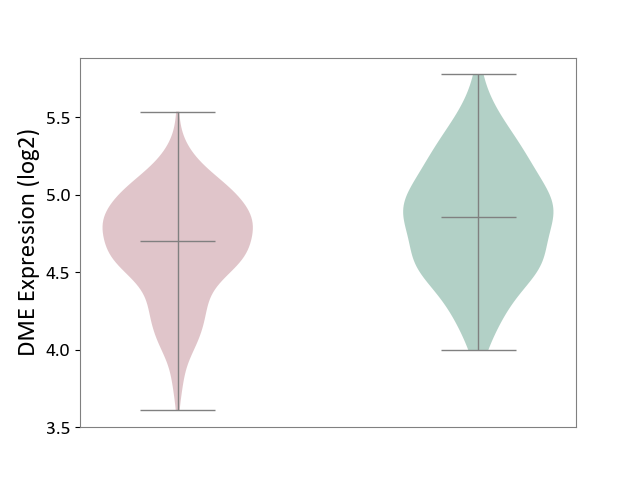
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 4A42 | Systemic sclerosis | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Scleroderma [ICD-11:4A42.Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.95E-01; Fold-change: -4.05E-02; Z-score: -2.35E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
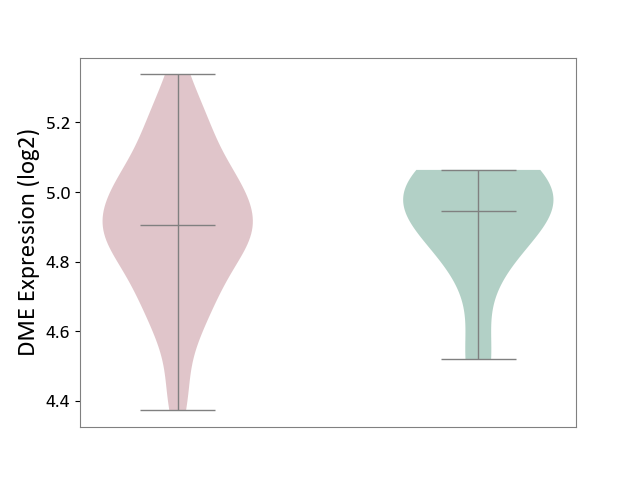
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 4A43 | Systemic autoimmune disease | Click to Show/Hide | |||
| The Studied Tissue | Salivary gland tissue | ||||
| The Specified Disease | Sjogren's syndrome [ICD-11:4A43.2] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.99E-01; Fold-change: -2.06E-01; Z-score: -1.70E+00 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 9.19E-02; Fold-change: -3.23E-01; Z-score: -1.53E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
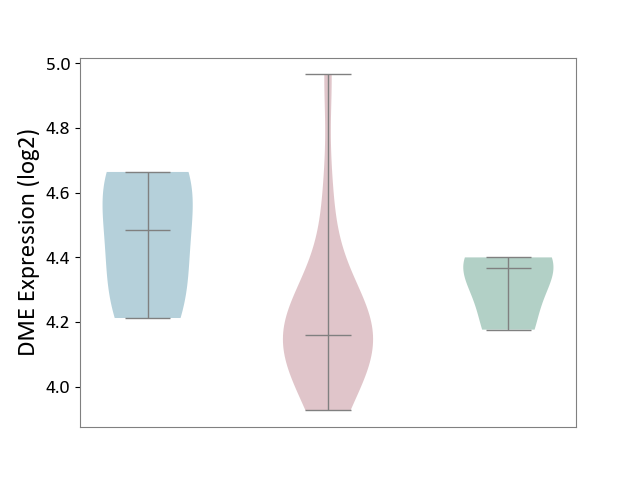
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 4A62 | Behcet disease | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Behcet's disease [ICD-11:4A62] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.52E-01; Fold-change: 4.31E-02; Z-score: 3.07E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
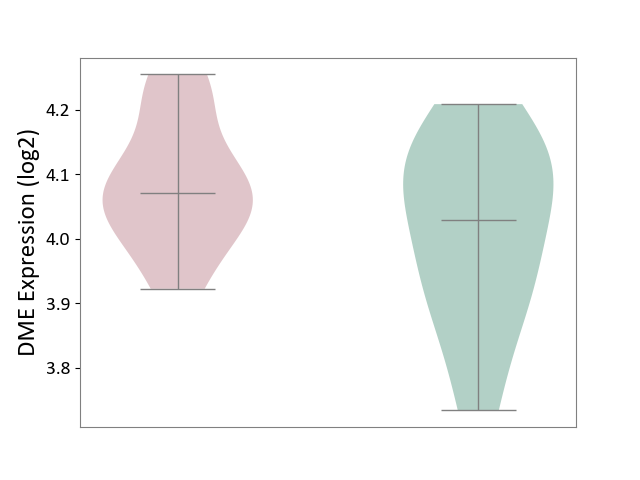
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 4B04 | Monocyte count disorder | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Autosomal dominant monocytopenia [ICD-11:4B04] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.87E-01; Fold-change: -6.16E-02; Z-score: -5.64E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
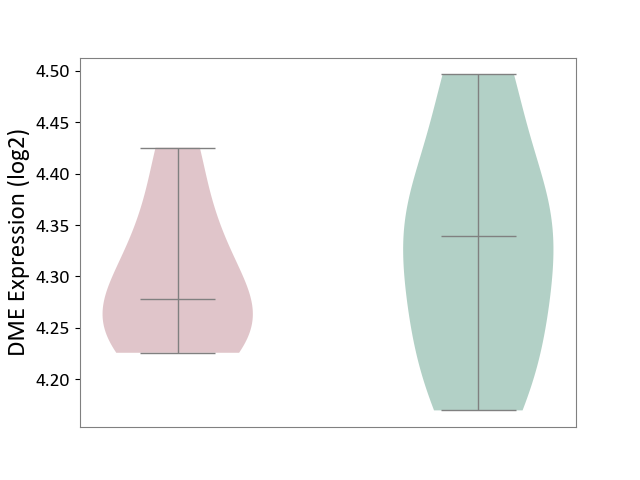
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 05 | Endocrine/nutritional/metabolic disease | Click to Show/Hide | |||
| ICD-11: 5A11 | Type 2 diabetes mellitus | Click to Show/Hide | |||
| The Studied Tissue | Omental adipose tissue | ||||
| The Specified Disease | Obesity related type 2 diabetes [ICD-11:5A11] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.38E-02; Fold-change: -1.12E-01; Z-score: -1.78E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |

|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Liver tissue | ||||
| The Specified Disease | Type 2 diabetes [ICD-11:5A11] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.76E-01; Fold-change: -4.64E-01; Z-score: -9.16E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
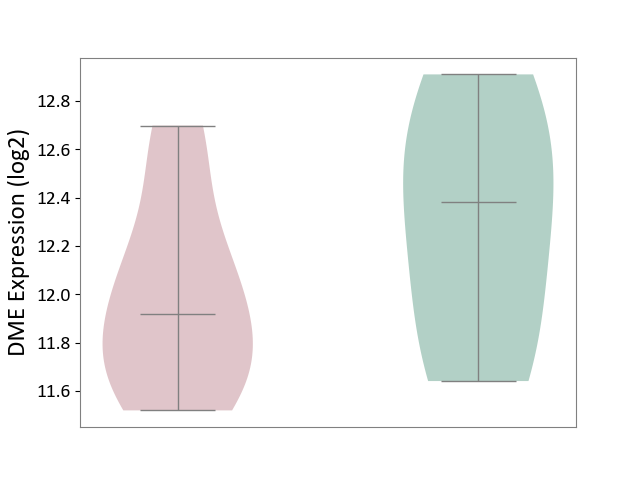
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 5A80 | Ovarian dysfunction | Click to Show/Hide | |||
| The Studied Tissue | Vastus lateralis muscle | ||||
| The Specified Disease | Polycystic ovary syndrome [ICD-11:5A80.1] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.86E-01; Fold-change: 7.97E-02; Z-score: 6.52E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
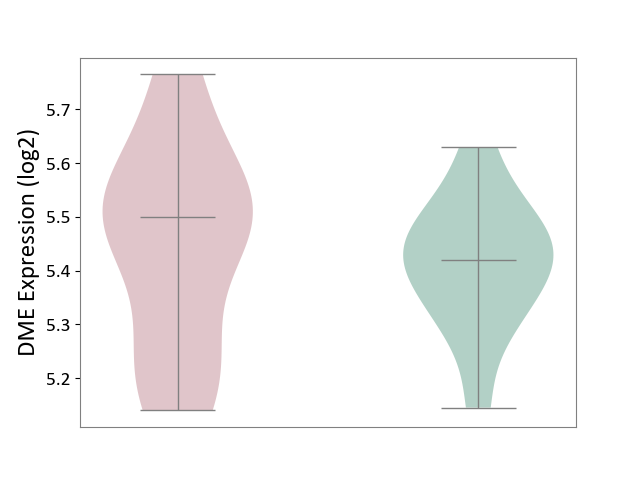
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 5C51 | Inborn carbohydrate metabolism disorder | Click to Show/Hide | |||
| The Studied Tissue | Biceps muscle | ||||
| The Specified Disease | Pompe disease [ICD-11:5C51.3] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.85E-01; Fold-change: -1.24E-01; Z-score: -8.96E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
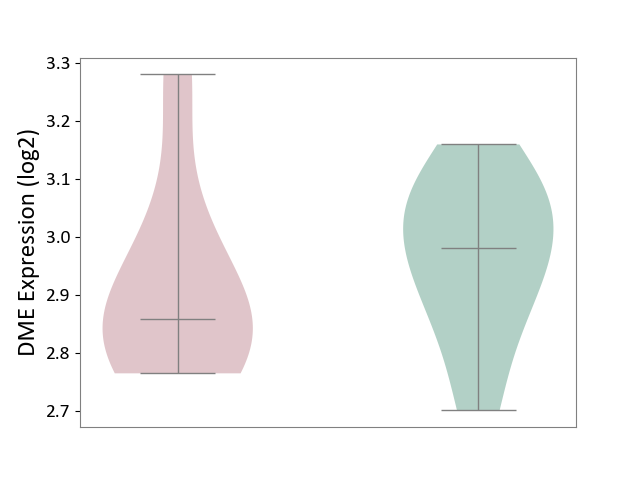
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 5C56 | Lysosomal disease | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Batten disease [ICD-11:5C56.1] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.89E-01; Fold-change: 1.66E-01; Z-score: 9.51E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
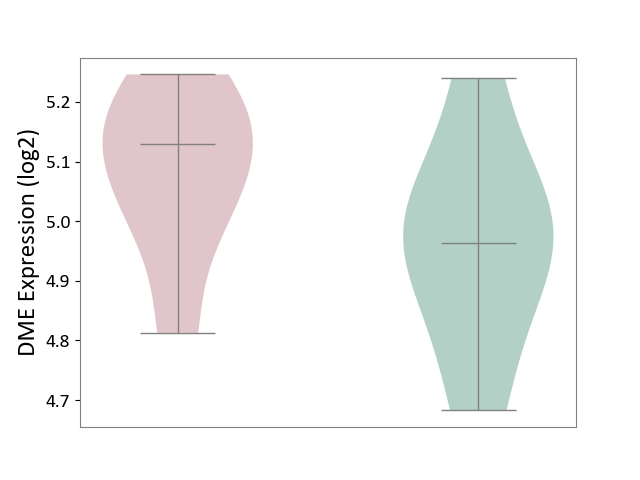
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 5C80 | Hyperlipoproteinaemia | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Familial hypercholesterolemia [ICD-11:5C80.00] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.08E-01; Fold-change: -8.08E-02; Z-score: -5.30E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
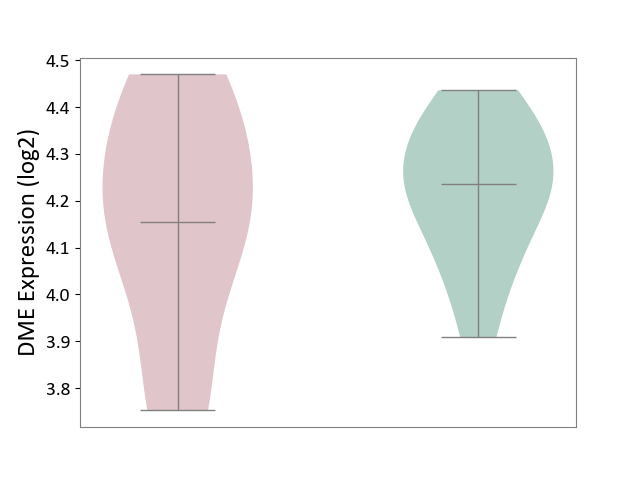
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Familial hypercholesterolemia [ICD-11:5C80.00] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.24E-05; Fold-change: -3.48E-01; Z-score: -1.34E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
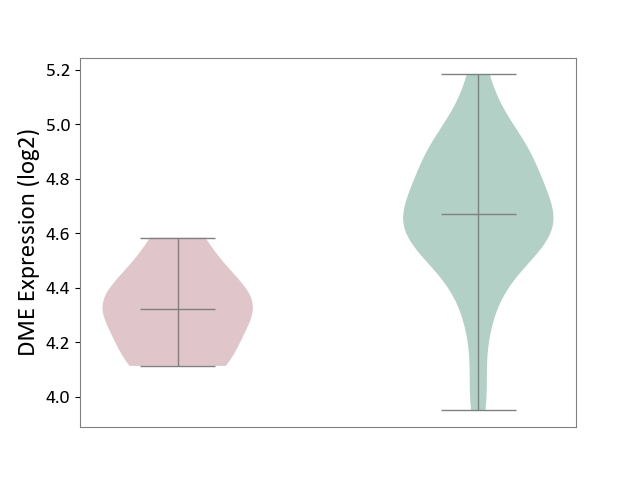
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 06 | Mental/behavioural/neurodevelopmental disorder | Click to Show/Hide | |||
| ICD-11: 6A02 | Autism spectrum disorder | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Autism [ICD-11:6A02] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.09E-01; Fold-change: -5.31E-02; Z-score: -2.65E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
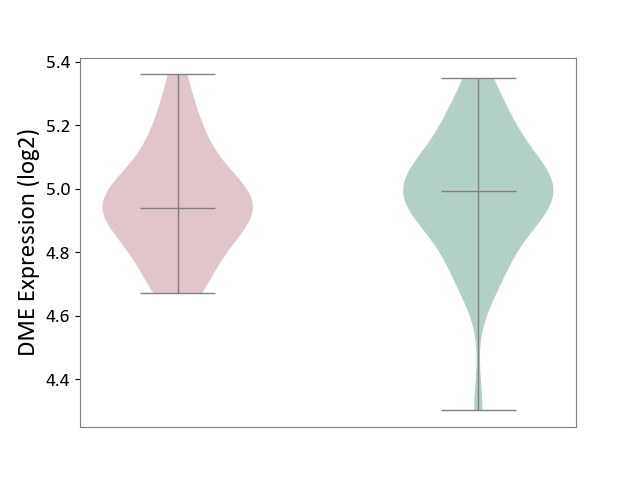
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 6A20 | Schizophrenia | Click to Show/Hide | |||
| The Studied Tissue | Prefrontal cortex | ||||
| The Specified Disease | Schizophrenia [ICD-11:6A20] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.66E-01; Fold-change: 4.25E-02; Z-score: 1.20E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
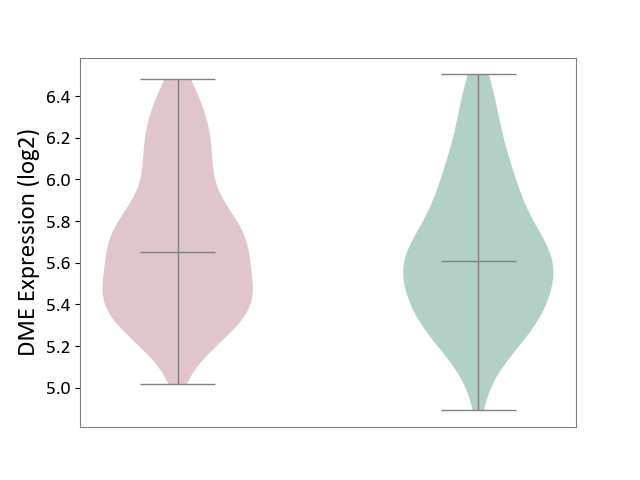
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Superior temporal cortex | ||||
| The Specified Disease | Schizophrenia [ICD-11:6A20] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.75E-01; Fold-change: 5.15E-02; Z-score: 3.64E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
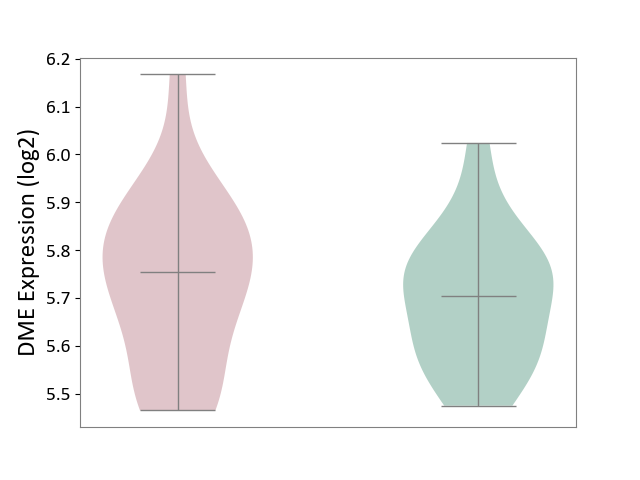
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 6A60 | Bipolar disorder | Click to Show/Hide | |||
| The Studied Tissue | Prefrontal cortex | ||||
| The Specified Disease | Bipolar disorder [ICD-11:6A60-6A6Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.31E-01; Fold-change: -4.19E-02; Z-score: -2.14E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
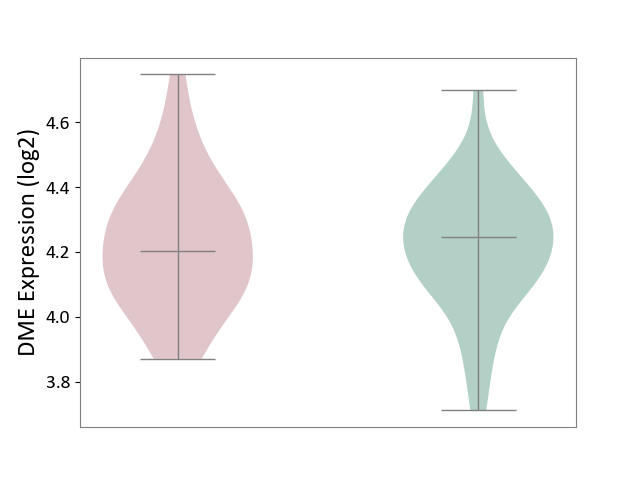
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 6A70 | Depressive disorder | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Major depressive disorder [ICD-11:6A70-6A7Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.17E-01; Fold-change: -5.18E-02; Z-score: -2.74E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
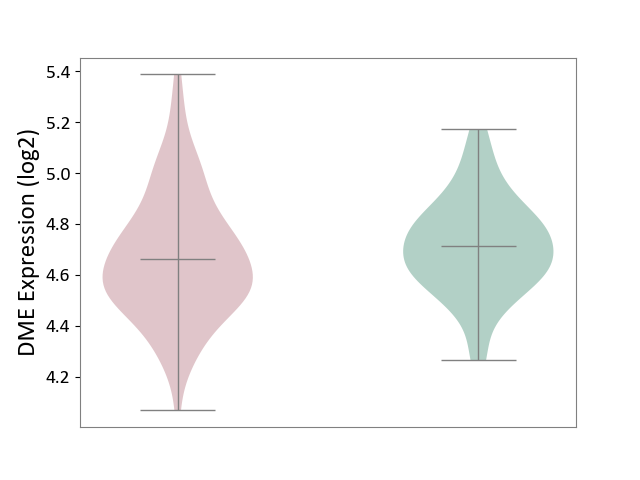
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Hippocampus | ||||
| The Specified Disease | Major depressive disorder [ICD-11:6A70-6A7Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.06E-01; Fold-change: -2.42E-02; Z-score: -1.64E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 08 | Nervous system disease | Click to Show/Hide | |||
| ICD-11: 8A00 | Parkinsonism | Click to Show/Hide | |||
| The Studied Tissue | Substantia nigra tissue | ||||
| The Specified Disease | Parkinson's disease [ICD-11:8A00.0] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.76E-01; Fold-change: 1.26E-01; Z-score: 8.27E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8A01 | Choreiform disorder | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Huntington's disease [ICD-11:8A01.10] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.43E-01; Fold-change: -2.48E-02; Z-score: -1.78E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8A20 | Alzheimer disease | Click to Show/Hide | |||
| The Studied Tissue | Entorhinal cortex | ||||
| The Specified Disease | Alzheimer's disease [ICD-11:8A20] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.04E-02; Fold-change: 6.29E-02; Z-score: 3.54E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |

|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8A2Y | Neurocognitive impairment | Click to Show/Hide | |||
| The Studied Tissue | White matter tissue | ||||
| The Specified Disease | HIV-associated neurocognitive impairment [ICD-11:8A2Y-8A2Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.40E-02; Fold-change: -1.70E-01; Z-score: -7.22E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8A40 | Multiple sclerosis | Click to Show/Hide | |||
| The Studied Tissue | Plasmacytoid dendritic cells | ||||
| The Specified Disease | Multiple sclerosis [ICD-11:8A40] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.85E-01; Fold-change: 2.24E-02; Z-score: 7.33E-02 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Spinal cord | ||||
| The Specified Disease | Multiple sclerosis [ICD-11:8A40] | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 6.29E-02; Fold-change: -1.88E-01; Z-score: -1.32E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8A60 | Epilepsy | Click to Show/Hide | |||
| The Studied Tissue | Peritumoral cortex tissue | ||||
| The Specified Disease | Epilepsy [ICD-11:8A60-8A6Z] | ||||
| The Expression Level of Disease Section Compare with the Other Disease Section | p-value: 1.10E-01; Fold-change: 1.59E-01; Z-score: 7.77E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in tissue other than the diseased tissue of patients
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Seizure [ICD-11:8A60-8A6Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.30E-01; Fold-change: -2.96E-02; Z-score: -1.60E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8B01 | Subarachnoid haemorrhage | Click to Show/Hide | |||
| The Studied Tissue | Intracranial artery | ||||
| The Specified Disease | Intracranial aneurysm [ICD-11:8B01.0] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.63E-01; Fold-change: 6.68E-02; Z-score: 3.45E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
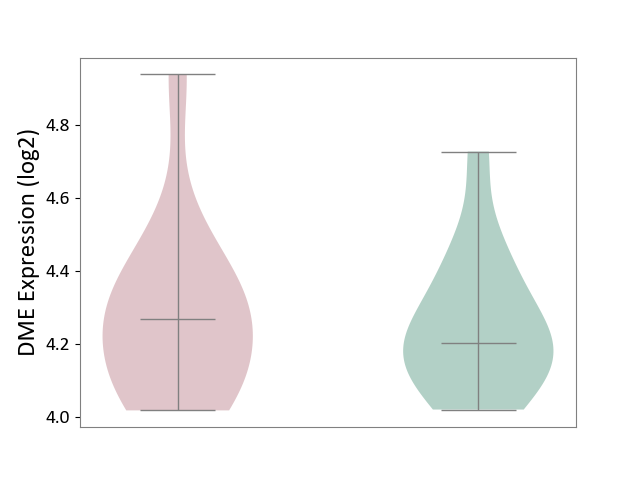
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8B11 | Cerebral ischaemic stroke | Click to Show/Hide | |||
| The Studied Tissue | Whole blood | ||||
| The Specified Disease | Cardioembolic stroke [ICD-11:8B11.20] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.87E-01; Fold-change: -1.13E-01; Z-score: -5.03E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
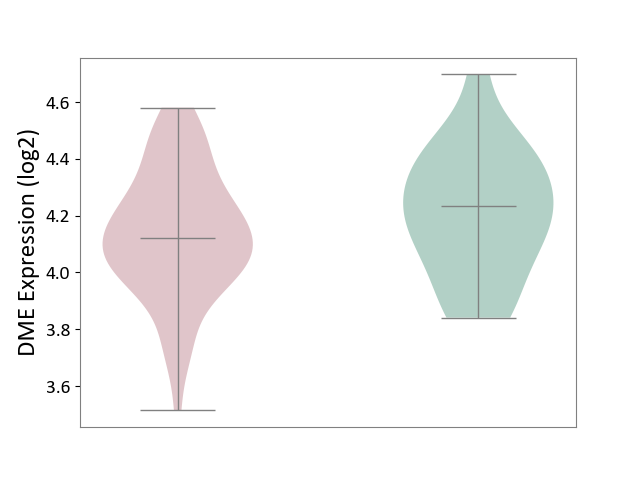
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Ischemic stroke [ICD-11:8B11] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.29E-02; Fold-change: 1.11E-01; Z-score: 6.58E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
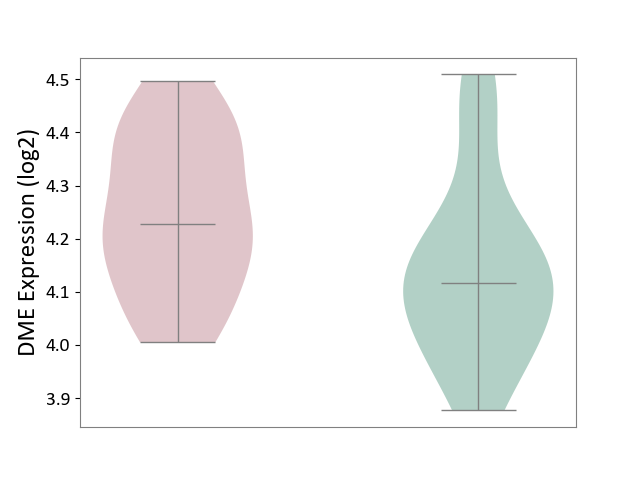
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8B60 | Motor neuron disease | Click to Show/Hide | |||
| The Studied Tissue | Skin | ||||
| The Specified Disease | Lateral sclerosis [ICD-11:8B60.4] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.18E-03; Fold-change: 2.30E-01; Z-score: 2.71E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
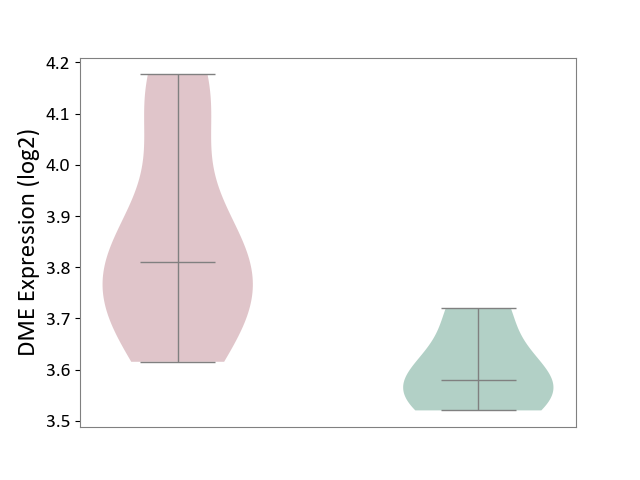
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Cervical spinal cord | ||||
| The Specified Disease | Lateral sclerosis [ICD-11:8B60.4] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.32E-01; Fold-change: 4.74E-02; Z-score: 1.73E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
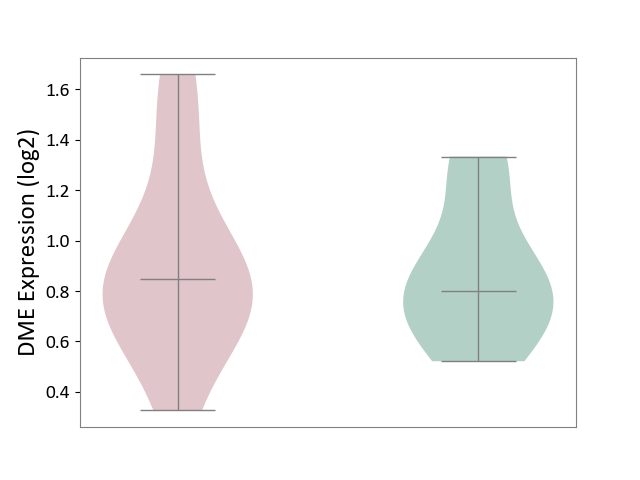
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8C70 | Muscular dystrophy | Click to Show/Hide | |||
| The Studied Tissue | Muscle tissue | ||||
| The Specified Disease | Myopathy [ICD-11:8C70.6] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.95E-02; Fold-change: -2.34E-01; Z-score: -1.05E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
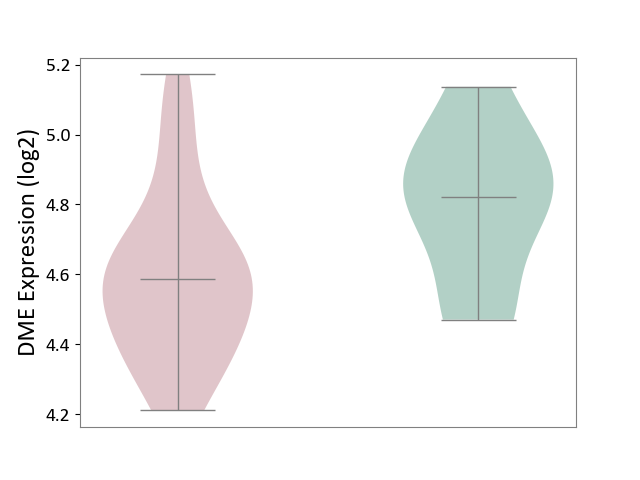
|
Click to View the Clearer Original Diagram | |||
| ICD-11: 8C75 | Distal myopathy | Click to Show/Hide | |||
| The Studied Tissue | Muscle tissue | ||||
| The Specified Disease | Tibial muscular dystrophy [ICD-11:8C75] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.93E-02; Fold-change: -8.20E-02; Z-score: -8.93E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
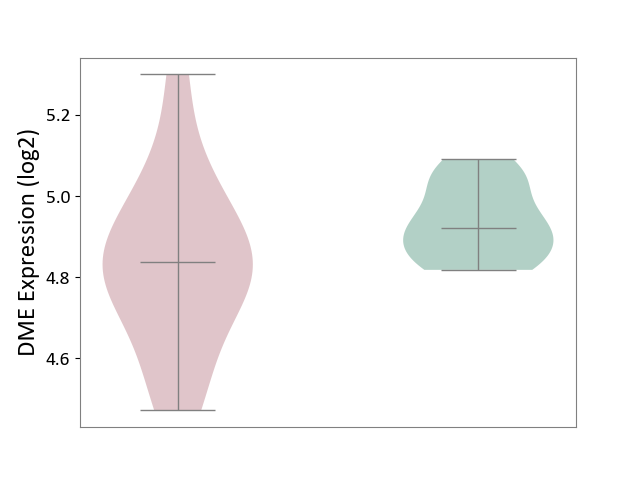
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 09 | Visual system disease | Click to Show/Hide | |||
| ICD-11: 9A96 | Anterior uveitis | Click to Show/Hide | |||
| The Studied Tissue | Peripheral monocyte | ||||
| The Specified Disease | Autoimmune uveitis [ICD-11:9A96] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.47E-01; Fold-change: -1.84E-01; Z-score: -9.58E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
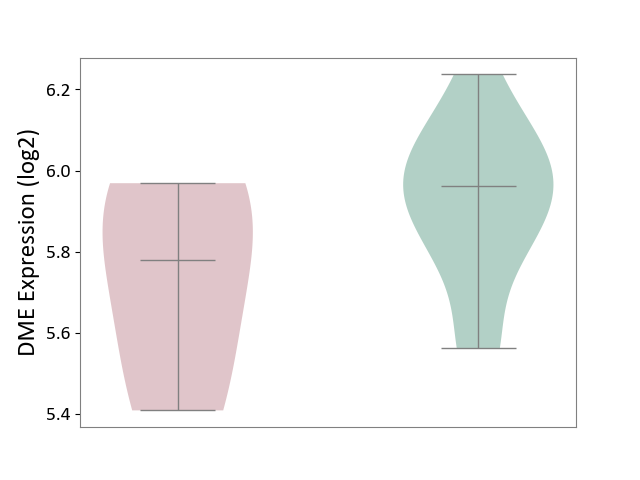
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 11 | Circulatory system disease | Click to Show/Hide | |||
| ICD-11: BA41 | Myocardial infarction | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Myocardial infarction [ICD-11:BA41-BA50] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.97E-01; Fold-change: 5.39E-02; Z-score: 1.24E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
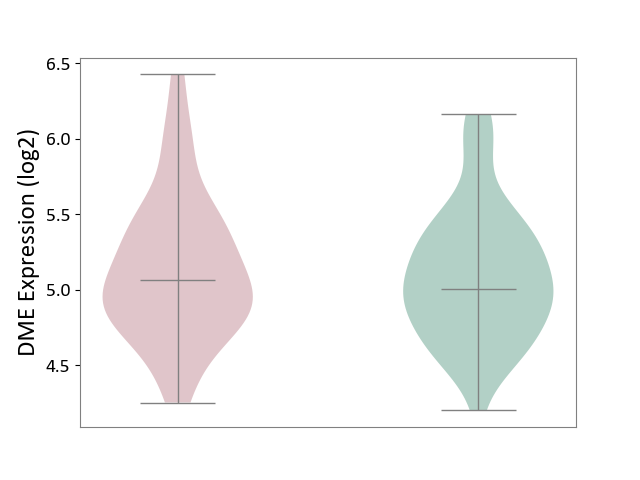
|
Click to View the Clearer Original Diagram | |||
| ICD-11: BA80 | Coronary artery disease | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Coronary artery disease [ICD-11:BA80-BA8Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.06E-01; Fold-change: -1.48E-01; Z-score: -8.48E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
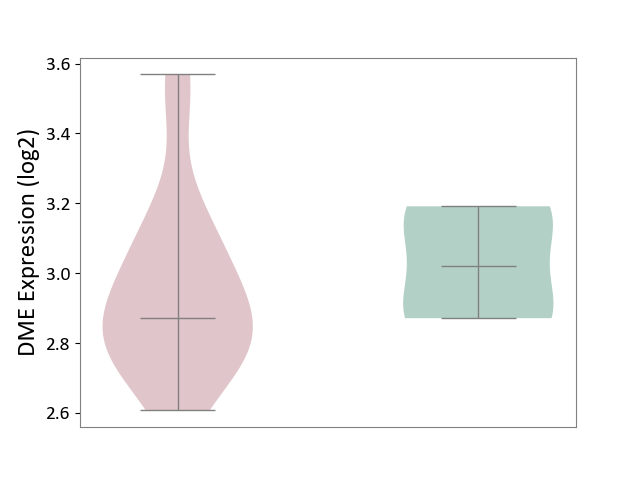
|
Click to View the Clearer Original Diagram | |||
| ICD-11: BB70 | Aortic valve stenosis | Click to Show/Hide | |||
| The Studied Tissue | Calcified aortic valve | ||||
| The Specified Disease | Aortic stenosis [ICD-11:BB70] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.46E-01; Fold-change: -1.10E-01; Z-score: -1.82E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
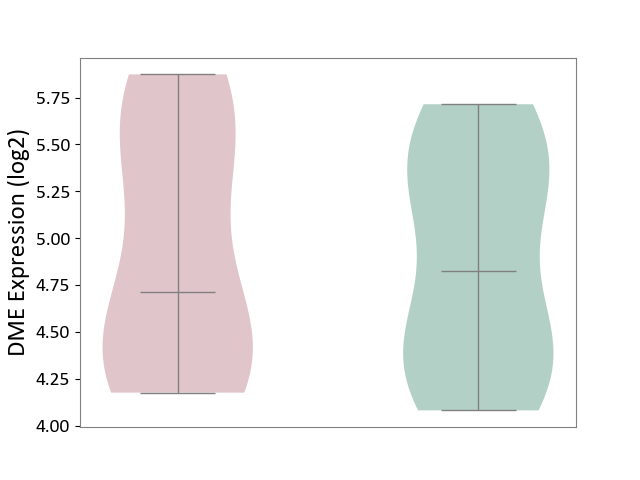
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 12 | Respiratory system disease | Click to Show/Hide | |||
| ICD-11: 7A40 | Central sleep apnoea | Click to Show/Hide | |||
| The Studied Tissue | Hyperplastic tonsil | ||||
| The Specified Disease | Apnea [ICD-11:7A40] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.97E-01; Fold-change: 2.63E-01; Z-score: 1.40E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
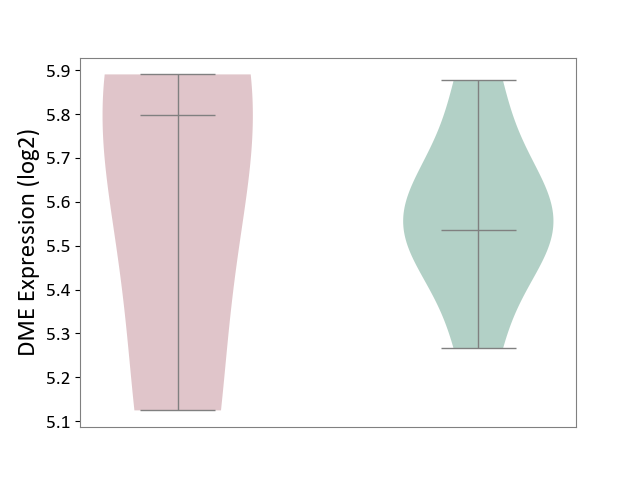
|
Click to View the Clearer Original Diagram | |||
| ICD-11: CA08 | Vasomotor or allergic rhinitis | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Olive pollen allergy [ICD-11:CA08.00] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.89E-01; Fold-change: 9.67E-02; Z-score: 4.76E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
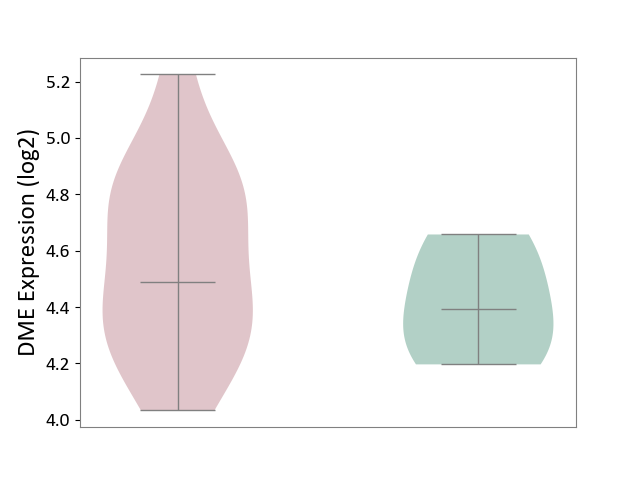
|
Click to View the Clearer Original Diagram | |||
| ICD-11: CA0A | Chronic rhinosinusitis | Click to Show/Hide | |||
| The Studied Tissue | Sinus mucosa tissue | ||||
| The Specified Disease | Chronic rhinosinusitis [ICD-11:CA0A] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.50E-01; Fold-change: 1.42E-01; Z-score: 1.16E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
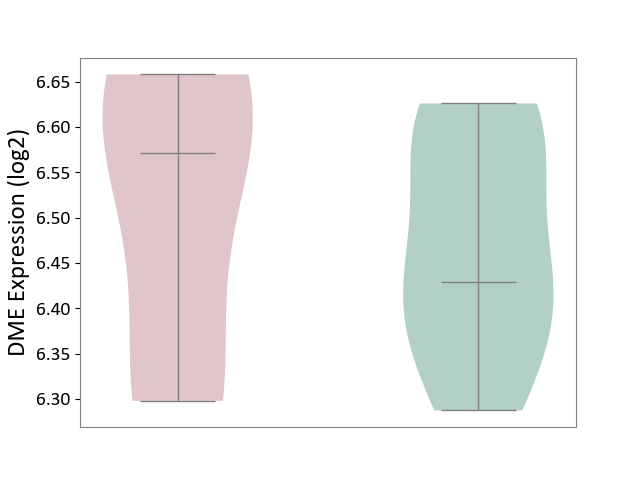
|
Click to View the Clearer Original Diagram | |||
| ICD-11: CA22 | Chronic obstructive pulmonary disease | Click to Show/Hide | |||
| The Studied Tissue | Lung tissue | ||||
| The Specified Disease | Chronic obstructive pulmonary disease [ICD-11:CA22] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.13E-01; Fold-change: 4.03E-02; Z-score: 2.50E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
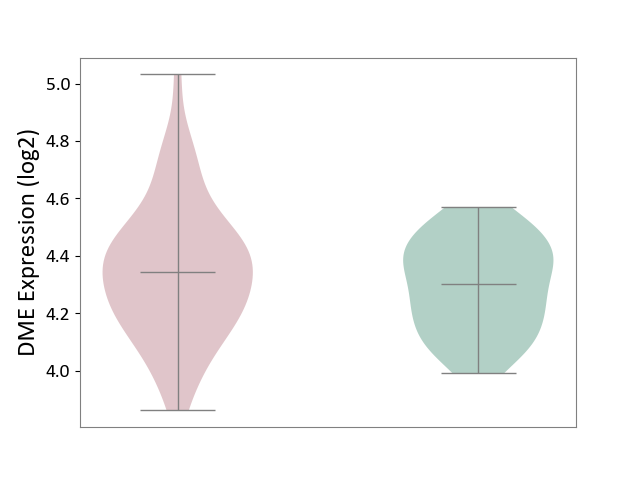
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Small airway epithelium | ||||
| The Specified Disease | Chronic obstructive pulmonary disease [ICD-11:CA22] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.21E-02; Fold-change: 8.17E-02; Z-score: 4.35E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
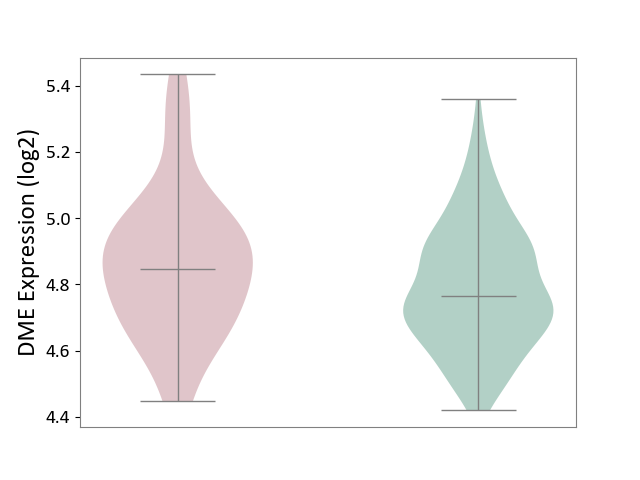
|
Click to View the Clearer Original Diagram | |||
| ICD-11: CA23 | Asthma | Click to Show/Hide | |||
| The Studied Tissue | Nasal and bronchial airway | ||||
| The Specified Disease | Asthma [ICD-11:CA23] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.90E-01; Fold-change: 2.35E-02; Z-score: 7.88E-02 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: CB03 | Idiopathic interstitial pneumonitis | Click to Show/Hide | |||
| The Studied Tissue | Lung tissue | ||||
| The Specified Disease | Idiopathic pulmonary fibrosis [ICD-11:CB03.4] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.36E-01; Fold-change: -2.34E-01; Z-score: -1.17E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 13 | Digestive system disease | Click to Show/Hide | |||
| ICD-11: DA0C | Periodontal disease | Click to Show/Hide | |||
| The Studied Tissue | Gingival tissue | ||||
| The Specified Disease | Periodontal disease [ICD-11:DA0C] | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.21E-02; Fold-change: -1.18E-01; Z-score: -4.76E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: DA42 | Gastritis | Click to Show/Hide | |||
| The Studied Tissue | Gastric antrum tissue | ||||
| The Specified Disease | Eosinophilic gastritis [ICD-11:DA42.2] | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.82E-01; Fold-change: -2.64E-01; Z-score: -4.06E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: DB92 | Non-alcoholic fatty liver disease | Click to Show/Hide | |||
| The Studied Tissue | Liver tissue | ||||
| The Specified Disease | Non-alcoholic fatty liver disease [ICD-11:DB92] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.18E-01; Fold-change: -6.23E-02; Z-score: -1.75E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
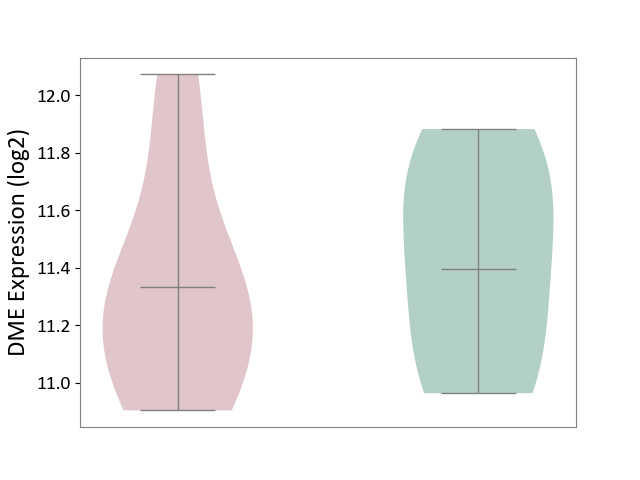
|
Click to View the Clearer Original Diagram | |||
| ICD-11: DB99 | Hepatic failure | Click to Show/Hide | |||
| The Studied Tissue | Liver tissue | ||||
| The Specified Disease | Liver failure [ICD-11:DB99.7-DB99.8] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.05E-04; Fold-change: -3.19E+00; Z-score: -2.66E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
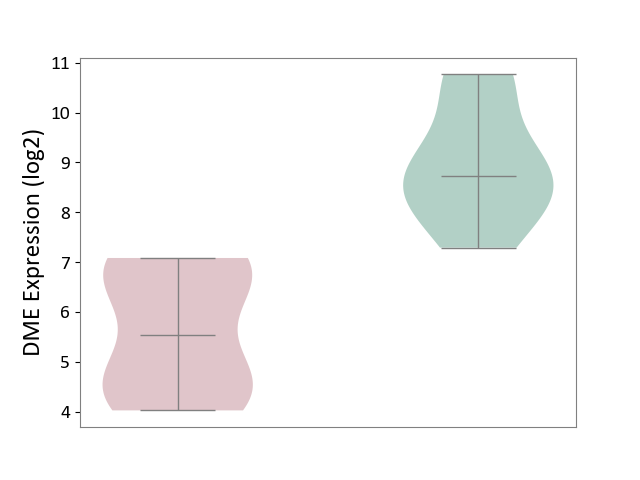
|
Click to View the Clearer Original Diagram | |||
| ICD-11: DD71 | Ulcerative colitis | Click to Show/Hide | |||
| The Studied Tissue | Colon mucosal tissue | ||||
| The Specified Disease | Ulcerative colitis [ICD-11:DD71] | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 2.75E-01; Fold-change: -2.15E-02; Z-score: -1.01E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in tissue other than the diseased tissue of patients
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
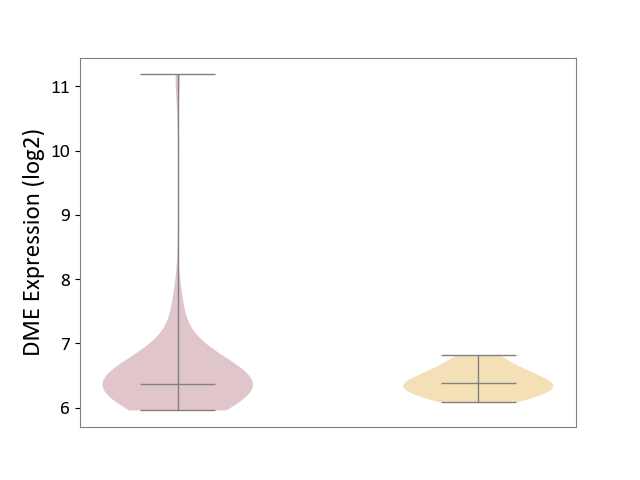
|
Click to View the Clearer Original Diagram | |||
| ICD-11: DD91 | Irritable bowel syndrome | Click to Show/Hide | |||
| The Studied Tissue | Rectal colon tissue | ||||
| The Specified Disease | Irritable bowel syndrome [ICD-11:DD91.0] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.40E-01; Fold-change: -2.17E-02; Z-score: -4.03E-02 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
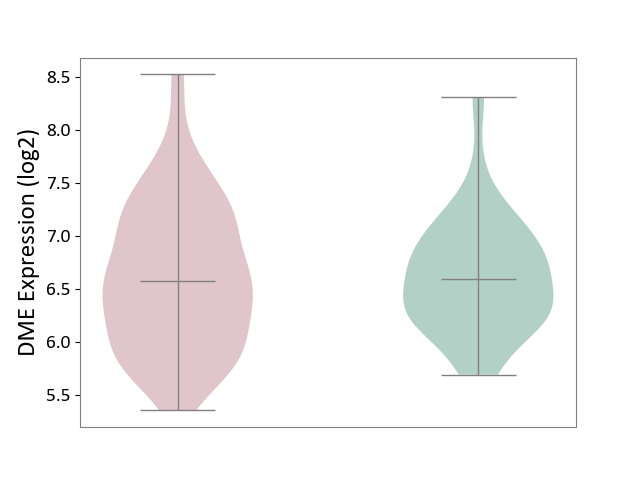
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 14 | Skin disease | Click to Show/Hide | |||
| ICD-11: EA80 | Atopic eczema | Click to Show/Hide | |||
| The Studied Tissue | Skin | ||||
| The Specified Disease | Atopic dermatitis [ICD-11:EA80] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.52E-04; Fold-change: 9.69E-02; Z-score: 7.57E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
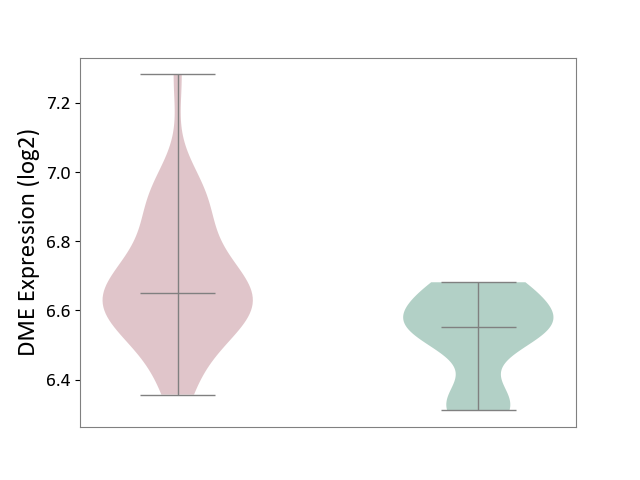
|
Click to View the Clearer Original Diagram | |||
| ICD-11: EA90 | Psoriasis | Click to Show/Hide | |||
| The Studied Tissue | Skin | ||||
| The Specified Disease | Psoriasis [ICD-11:EA90] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.67E-01; Fold-change: -3.83E-03; Z-score: -1.24E-02 | ||||
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.52E-19; Fold-change: -4.66E-01; Z-score: -1.22E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue adjacent to the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
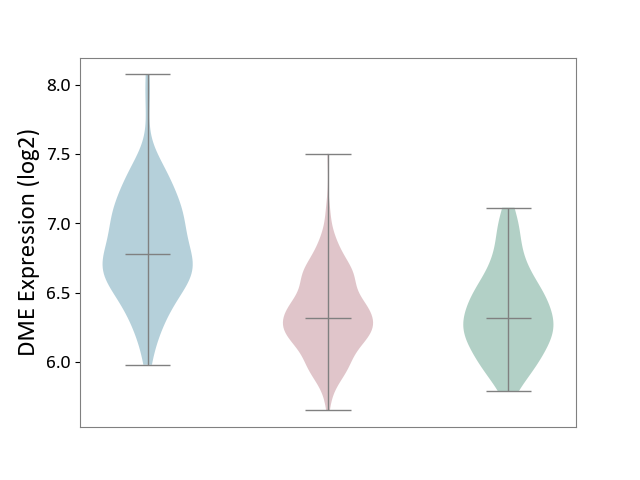
|
Click to View the Clearer Original Diagram | |||
| ICD-11: ED63 | Acquired hypomelanotic disorder | Click to Show/Hide | |||
| The Studied Tissue | Skin | ||||
| The Specified Disease | Vitiligo [ICD-11:ED63.0] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.70E-01; Fold-change: 2.10E-01; Z-score: 5.81E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
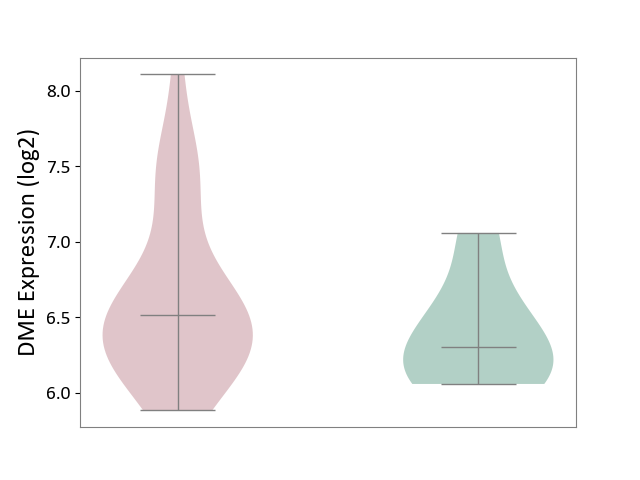
|
Click to View the Clearer Original Diagram | |||
| ICD-11: ED70 | Alopecia or hair loss | Click to Show/Hide | |||
| The Studied Tissue | Skin from scalp | ||||
| The Specified Disease | Alopecia [ICD-11:ED70] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.73E-01; Fold-change: -3.42E-03; Z-score: -9.10E-03 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
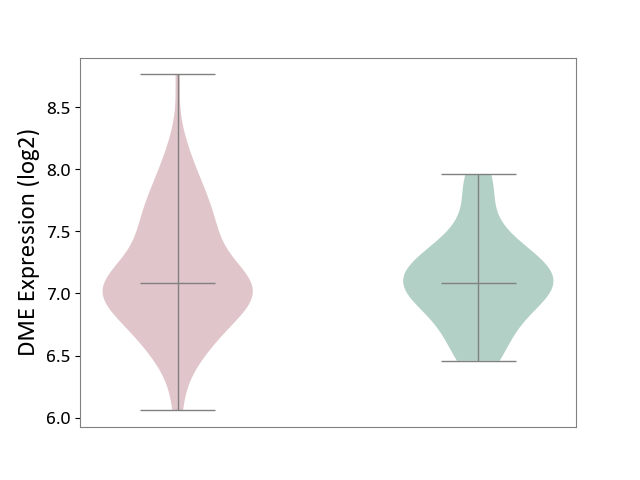
|
Click to View the Clearer Original Diagram | |||
| ICD-11: EK0Z | Contact dermatitis | Click to Show/Hide | |||
| The Studied Tissue | Skin | ||||
| The Specified Disease | Sensitive skin [ICD-11:EK0Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.32E-02; Fold-change: -3.50E-01; Z-score: -1.37E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
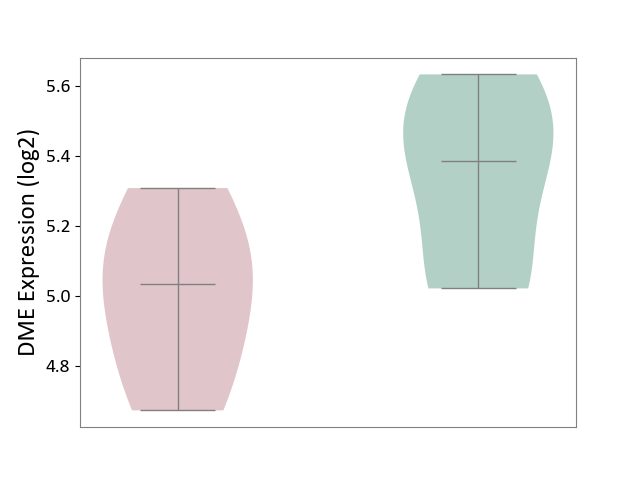
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 15 | Musculoskeletal system/connective tissue disease | Click to Show/Hide | |||
| ICD-11: FA00 | Osteoarthritis | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Arthropathy [ICD-11:FA00-FA5Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.22E-01; Fold-change: 6.75E-02; Z-score: 4.19E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
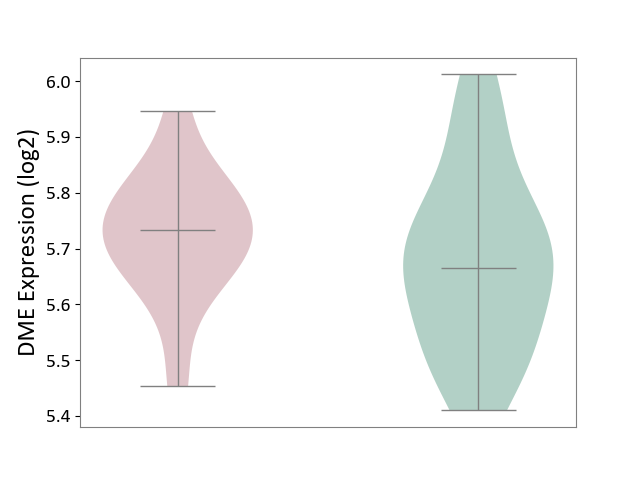
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Synovial tissue | ||||
| The Specified Disease | Osteoarthritis [ICD-11:FA00-FA0Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.33E-01; Fold-change: 1.48E-01; Z-score: 3.59E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
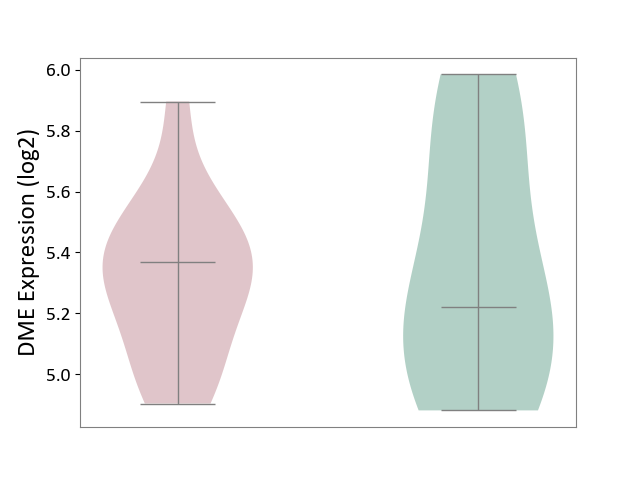
|
Click to View the Clearer Original Diagram | |||
| ICD-11: FA20 | Rheumatoid arthritis | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Childhood onset rheumatic disease [ICD-11:FA20.Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.83E-01; Fold-change: -1.32E-02; Z-score: -4.74E-02 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
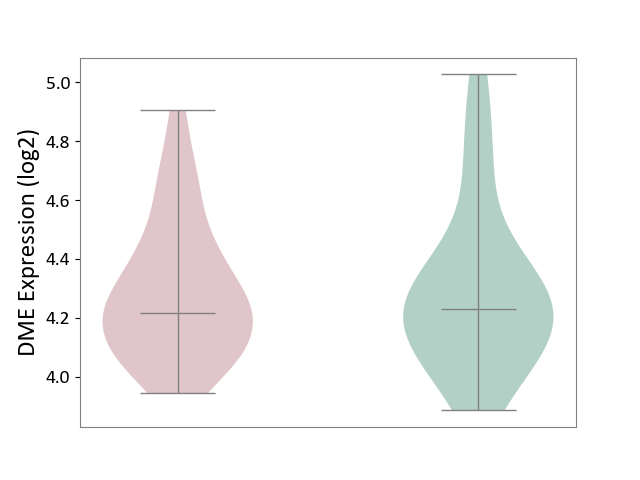
|
Click to View the Clearer Original Diagram | |||
| The Studied Tissue | Synovial tissue | ||||
| The Specified Disease | Rheumatoid arthritis [ICD-11:FA20] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.81E-01; Fold-change: 2.72E-01; Z-score: 6.95E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: FA24 | Juvenile idiopathic arthritis | Click to Show/Hide | |||
| The Studied Tissue | Peripheral blood | ||||
| The Specified Disease | Juvenile idiopathic arthritis [ICD-11:FA24] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.39E-03; Fold-change: 1.17E-01; Z-score: 4.33E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: FA92 | Inflammatory spondyloarthritis | Click to Show/Hide | |||
| The Studied Tissue | Pheripheral blood | ||||
| The Specified Disease | Ankylosing spondylitis [ICD-11:FA92.0Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.78E-01; Fold-change: -5.92E-02; Z-score: -3.47E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD-11: FB83 | Low bone mass disorder | Click to Show/Hide | |||
| The Studied Tissue | Bone marrow | ||||
| The Specified Disease | Osteoporosis [ICD-11:FB83.1] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 8.60E-02; Fold-change: 1.58E-01; Z-score: 1.36E+00 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 16 | Genitourinary system disease | Click to Show/Hide | |||
| ICD-11: GA10 | Endometriosis | Click to Show/Hide | |||
| The Studied Tissue | Endometrium tissue | ||||
| The Specified Disease | Endometriosis [ICD-11:GA10] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.50E-01; Fold-change: 8.27E-02; Z-score: 3.14E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
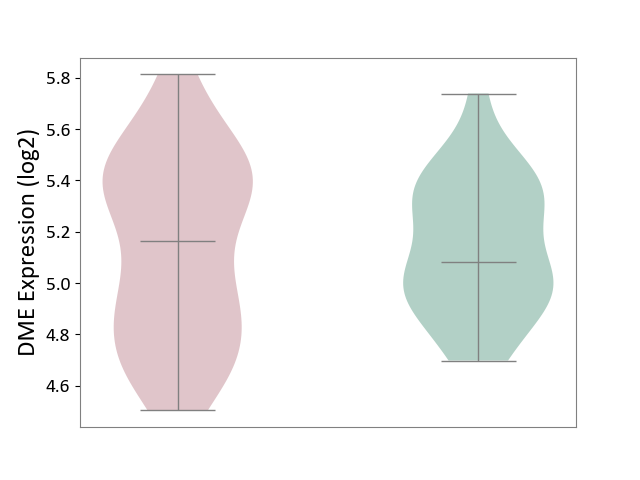
|
Click to View the Clearer Original Diagram | |||
| ICD-11: GC00 | Cystitis | Click to Show/Hide | |||
| The Studied Tissue | Bladder tissue | ||||
| The Specified Disease | Interstitial cystitis [ICD-11:GC00.3] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.87E-01; Fold-change: -4.14E-02; Z-score: -5.17E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
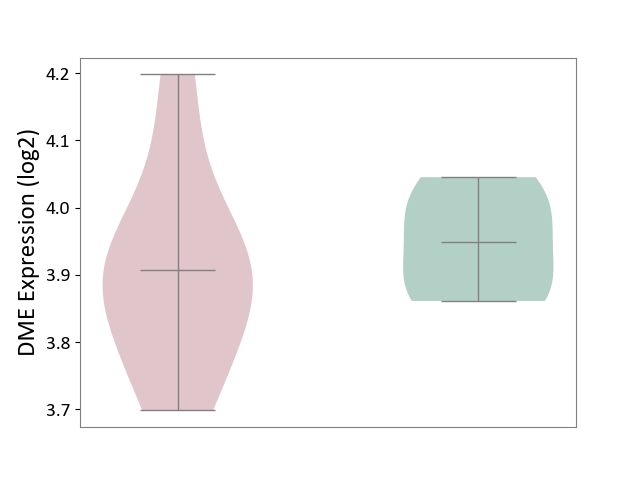
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 19 | Condition originating in perinatal period | Click to Show/Hide | |||
| ICD-11: KA21 | Short gestation disorder | Click to Show/Hide | |||
| The Studied Tissue | Myometrium | ||||
| The Specified Disease | Preterm birth [ICD-11:KA21.4Z] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.76E-01; Fold-change: -2.60E-02; Z-score: -3.37E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
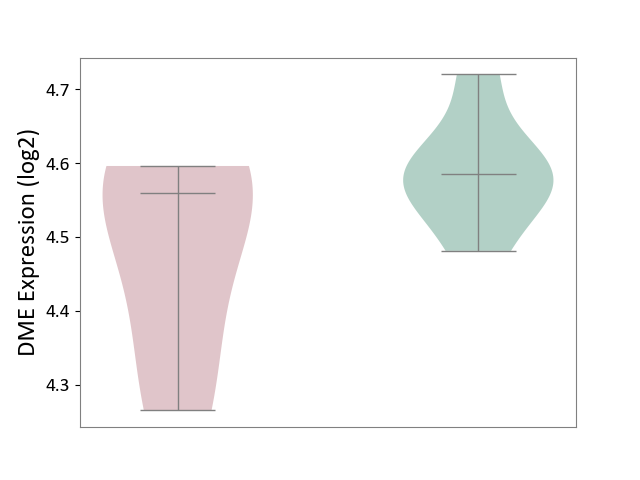
|
Click to View the Clearer Original Diagram | |||
| ICD Disease Classification 20 | Developmental anomaly | Click to Show/Hide | |||
| ICD-11: LD2C | Overgrowth syndrome | Click to Show/Hide | |||
| The Studied Tissue | Adipose tissue | ||||
| The Specified Disease | Simpson golabi behmel syndrome [ICD-11:LD2C] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 6.80E-01; Fold-change: -9.44E-03; Z-score: -8.86E-02 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
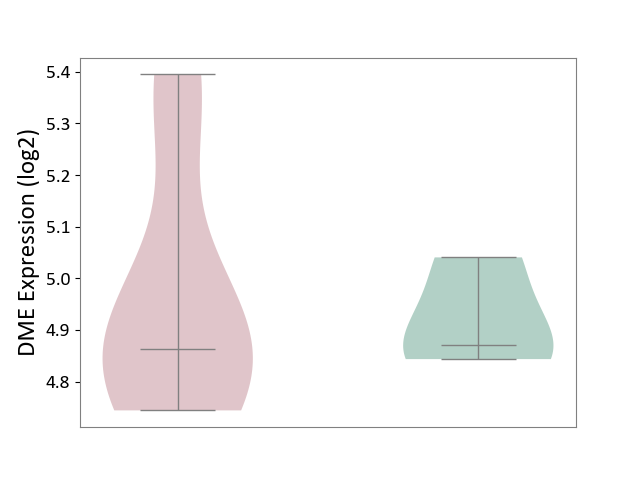
|
Click to View the Clearer Original Diagram | |||
| ICD-11: LD2D | Phakomatoses/hamartoneoplastic syndrome | Click to Show/Hide | |||
| The Studied Tissue | Perituberal tissue | ||||
| The Specified Disease | Tuberous sclerosis complex [ICD-11:LD2D.2] | ||||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.01E-01; Fold-change: 6.22E-02; Z-score: 5.20E-01 | ||||
|
DME expression in the diseased tissue of patients
DME expression in the normal tissue of healthy individuals
|
|||||
| Violin Diagram of DME Disease-specific Protein Abundances |
|
Click to View the Clearer Original Diagram | |||
| References | |||||
|---|---|---|---|---|---|
| 1 | Cobicistat versus ritonavir boosting and differences in the drug-drug interaction profiles with co-medications. J Antimicrob Chemother. 2016 Jul;71(7):1755-8. | ||||
| 2 | Drug-drug interaction potential of darolutamide: in vitro and clinical studies. Eur J Drug Metab Pharmacokinet. 2019 Dec;44(6):747-759. | ||||
| 3 | DrugBank(Pharmacology-Metabolism)Bupropion | ||||
| 4 | Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004 Sep;310(3):1062-75. | ||||
| 5 | Metabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor apatinib in humans | ||||
| 6 | In vitro assessment of time-dependent inhibitory effects on CYP2C8 and CYP3A activity by fourteen protein kinase inhibitors. Drug Metab Dispos. 2014 Jul;42(7):1202-9. | ||||
| 7 | Effect of alosetron on the pharmacokinetics of alprazolam. J Clin Pharmacol. 2001 Apr;41(4):452-4. | ||||
| 8 | Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans | ||||
| 9 | Pharmacokinetics, metabolism and excretion of radiolabeled fostemsavir administered with or without ritonavir in healthy male subjects | ||||
| 10 | Pharmacokinetics of Temsavir, the Active Moiety of the HIV-1 Attachment Inhibitor Prodrug, Fostemsavir, Coadministered with Cobicistat, Etravirine, Darunavir/Cobicistat, or Darunavir/Ritonavir with or without Etravirine in Healthy Participants | ||||
| 11 | FDA label of Idelalisib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 12 | Contribution of three CYP3A isoforms to metabolism of R- and S-warfarin. Drug Metab Lett. 2010 Dec;4(4):213-9. | ||||
| 13 | A potential role for the estrogen-metabolizing cytochrome P450 enzymes in human breast carcinogenesis. Breast Cancer Res Treat. 2003 Dec;82(3):191-7. | ||||
| 14 | Regioselective hydroxylation of steroid hormones by human cytochromes P450. Drug Metab Rev. 2015 May;47(2):89-110. | ||||
| 15 | Roles of CYP2A6 and CYP2B6 in nicotine C-oxidation by human liver microsomes. Arch Toxicol. 1999 Mar;73(2):65-70. | ||||
| 16 | Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor: a novel treatment option for nurse practitioners treating patients with psoriatic disease. J Am Assoc Nurse Pract. 2016 Dec;28(12):683-695. | ||||
| 17 | Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005 Jun;33(6):764-70. | ||||
| 18 | Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448. | ||||
| 19 | Progress with palbociclib in breast cancer: latest evidence and clinical considerations. Ther Adv Med Oncol. 2017 Feb;9(2):83-105. | ||||
| 20 | PubChem:Pimavanserin | ||||
| 21 | Apalutamide: first global approval. Drugs. 2018 Apr;78(6):699-705. | ||||
| 22 | Absorption, Distribution, Metabolism, and Excretion of Capmatinib (INC280) in Healthy Male Volunteers and In Vitro Aldehyde Oxidase Phenotyping of the Major Metabolite | ||||
| 23 | Pharmacokinetics of capmatinib in participants with hepatic impairment: A phase 1, open-label, single-dose, parallel-group study | ||||
| 24 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | ||||
| 25 | Absorption, Metabolism, and Excretion, In Vitro Pharmacology, and Clinical Pharmacokinetics of Ozanimod, a Novel Sphingosine 1-Phosphate Receptor Modulator | ||||
| 26 | Enhanced elimination of betamethasone in dichorionic twin pregnancies | ||||
| 27 | Relative contributions of the five major human cytochromes P450, 1A2, 2C9, 2C19, 2D6, and 3A4, to the hepatic metabolism of the proteasome inhibitor bortezomib. Drug Metab Dispos. 2005 Nov;33(11):1723-8. | ||||
| 28 | Daclatasvir: a NS5A replication complex inhibitor for hepatitis C infection. Ann Pharmacother. 2016 Jan;50(1):39-46. | ||||
| 29 | Delavirdine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2001;40(3):207-26. | ||||
| 30 | Cytochrome P450 3A4-mediated interaction of diclofenac and quinidine. Drug Metab Dispos. 2000 Sep;28(9):1043-50. | ||||
| 31 | CYP2D6 and CYP3A4 involvement in the primary oxidative metabolism of hydrocodone by human liver microsomes. Br J Clin Pharmacol. 2004 Mar;57(3):287-97. | ||||
| 32 | Pharmacokinetic and pharmacoimmunodynamic interactions between prednisolone and sirolimus in adrenalectomized rats. J Pharmacokinet Biopharm. 1999 Feb;27(1):1-21. | ||||
| 33 | Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation. Drug Metab Dispos. 2014 Feb;42(2):245-9. | ||||
| 34 | Clinical pharmacokinetics of galantamine. Clin Pharmacokinet. 2003;42(15):1383-92. | ||||
| 35 | Absorption, metabolism, and excretion of oral 14C radiolabeled ibrutinib: an open-label, phase I, single-dose study in healthy men. Drug Metab Dispos. 2015 Feb;43(2):289-97. | ||||
| 36 | The metabolism of amiodarone by various CYP isoenzymes of human and rat, and the inhibitory influence of ketoconazole. J Pharm Pharm Sci. 2008;11(1):147-59. | ||||
| 37 | Exploring the metabolism of loxoprofen in liver microsomes: the role of cytochrome P450 and UDP-glucuronosyltransferase in its biotransformation. Pharmaceutics. 2018 Aug 2;10(3). pii: E112. | ||||
| 38 | Metoclopramide is metabolized by CYP2D6 and is a reversible inhibitor, but not inactivator, of CYP2D6. Xenobiotica. 2014 Apr;44(4):309-319. | ||||
| 39 | Buprenorphine in cancer pain. Support Care Cancer. 2005 Nov;13(11):878-87. | ||||
| 40 | Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur J Clin Pharmacol. 2003 Sep;59(5-6):429-42. | ||||
| 41 | Contribution of human hepatic cytochrome P450 isoforms to regioselective hydroxylation of steroid hormones. Xenobiotica. 1998 Jun;28(6):539-47. | ||||
| 42 | Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics of pioglitazone. Clin Pharmacol Ther. 2005 May;77(5):404-14. | ||||
| 43 | FDA label of Acalabrutinib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 44 | Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem. 2009;16(31):4066-218. | ||||
| 45 | Clinical pharmacokinetics of almotriptan, a serotonin 5-HT(1B/1D) receptor agonist for the treatment of migraine. Clin Pharmacokinet. 2005;44(3):237-46. | ||||
| 46 | Identification of the human liver enzymes involved in the metabolism of the antimigraine agent almotriptan. Drug Metab Dispos. 2003 Apr;31(4):404-11. | ||||
| 47 | Crizotinib for the treatment of non-small-cell lung cancer. Am J Health Syst Pharm. 2013 Jun 1;70(11):943-7. | ||||
| 48 | Potent mechanism-based inhibition of CYP3A4 by imatinib explains its liability to interact with CYP3A4 substrates. Br J Pharmacol. 2012 Apr;165(8):2787-98. | ||||
| 49 | Pharmacokinetics of lidocaine hydrochloride metabolized by CYP3A4 in Chinese Han volunteers living at low altitude and in native Han and Tibetan Chinese volunteers living at high altitude. Pharmacology. 2016;97(3-4):107-13. | ||||
| 50 | Clinical pharmacokinetics and pharmacodynamics of linagliptin Clin Pharmacokinet. 2012 Jul 1;51(7):411-27. doi: 10.2165/11630900-000000000-00000. | ||||
| 51 | The metabolism and disposition of the oral dipeptidyl peptidase-4 inhibitor, linagliptin, in humans Drug Metab Dispos. 2010 Apr;38(4):667-78. doi: 10.1124/dmd.109.031476. | ||||
| 52 | Does metronidazole interact with CYP3A substrates by inhibiting their metabolism through this metabolic pathway? Or should other mechanisms be considered? Ann Pharmacother. 2007 Apr;41(4):653-8. | ||||
| 53 | Drug Interactions Flockhart Table | ||||
| 54 | Simultaneous assessment of drug interactions with low- and high-extraction opioids: application to parecoxib effects on the pharmacokinetics and pharmacodynamics of fentanyl and alfentanil. Anesthesiology. 2003 Apr;98(4):853-61. | ||||
| 55 | Effect of gemfibrozil on the metabolism of brivaracetam in vitro and in human subjects. Drug Metab Dispos. 2012 Aug;40(8):1466-72. | ||||
| 56 | Cytochrome P450-Catalyzed Metabolism of Cannabidiol to the Active Metabolite 7-Hydroxy-Cannabidiol Drug Metab Dispos. 2021 Oct;49(10):882-891. doi: 10.1124/dmd.120.000350. | ||||
| 57 | Metabolism of capsaicin by cytochrome P450 produces novel dehydrogenated metabolites and decreases cytotoxicity to lung and liver cells. Chem Res Toxicol. 2003 Mar;16(3):336-49. | ||||
| 58 | Dasatinib significantly reduced in vivo exposure to cyclosporine in a rat model: The possible involvement of CYP3A induction. Pharmacol Rep. 2019 Apr;71(2):201-205. | ||||
| 59 | CYP3A4*18B and CYP3A5*3 polymorphisms contribute to pharmacokinetic variability of cyclosporine among healthy Chinese subjects. Eur J Pharm Sci. 2015 Aug 30;76:238-44. | ||||
| 60 | Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007 Jul;151(6):737-48. | ||||
| 61 | Characterisation of the absorption, distribution, metabolism, excretion and mass balance of doravirine, a non-nucleoside reverse transcriptase inhibitor in humans. Xenobiotica. 2019 Apr;49(4):422-432. | ||||
| 62 | Priapism induced by boceprevir-CYP3A4 inhibition and alpha-adrenergic blockade: case report. Clin Infect Dis. 2014 Jan;58(1):e35-8. | ||||
| 63 | Rifampicin, a keystone inducer of drug metabolism: from Herbert Remmer's pioneering ideas to modern concepts. Drug Metab Rev. 2004 Oct;36(3-4):497-509. | ||||
| 64 | Effects of strong and moderate CYP3A4 inducers on the pharmacokinetics of fedratinib in healthy adult participants | ||||
| 65 | In vivo Pharmacokinetic Drug-Drug Interaction Studies Between Fedratinib and Antifungal Agents Based on a Newly Developed and Validated UPLC/MS-MS Method Front Pharmacol. 2021 Feb 5;11:626897. doi: 10.3389/fphar.2020.626897. | ||||
| 66 | Drug interactions with irbesartan. Clin Pharmacokinet. 2001;40(8):605-14. | ||||
| 67 | Mechanism-based inactivation of cytochrome P450 3A4 by lapatinib. Mol Pharmacol. 2010 Oct;78(4):693-703. | ||||
| 68 | Influence of CYP3A4/5 and ABC transporter polymorphisms on lenvatinib plasma trough concentrations in Japanese patients with thyroid cancer. Sci Rep. 2019 Apr 1;9(1):5404. | ||||
| 69 | PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics. 2014 Jan;24(1):73-9. | ||||
| 70 | CYP2C76-mediated species difference in drug metabolism: a comparison of pitavastatin metabolism between monkeys and humans | ||||
| 71 | Silodosin for benign prostatic hyperplasia. Ann Pharmacother. 2010 Feb;44(2):302-10. | ||||
| 72 | Metabolism and disposition of siponimod, a novel selective S1P1/S1P5 agonist, in healthy volunteers and in vitro identification of human cytochrome P450 enzymes involved in its oxidative metabolism. Drug Metab Dispos. 2018 Jul;46(7):1001-1013. | ||||
| 73 | Cytochromes P450 1A2 and 3A4 Catalyze the Metabolic Activation of Sunitinib | ||||
| 74 | A phenotype-genotype approach to predicting CYP450 and P-glycoprotein drug interactions with the mixed inhibitor/inducer tipranavir/ritonavir. Clin Pharmacol Ther. 2010 Jun;87(6):735-42. | ||||
| 75 | Identification of human cytochrome P450s involved in the formation of all-trans-retinoic acid principal metabolites. Mol Pharmacol. 2000 Dec;58(6):1341-8. | ||||
| 76 | Lack of effect of aprepitant on the pharmacokinetics of docetaxel in cancer patients. Cancer Chemother Pharmacol. 2005 Jun;55(6):609-16. | ||||
| 77 | Asenapine for the treatment of manic and mixed episodes associated with bipolar I disorder: from clinical research to clinical practice. Expert Opin Pharmacother. 2013 Mar;14(4):489-504. | ||||
| 78 | Brentuximab vedotin. | ||||
| 79 | Oxidative metabolism of bupivacaine into pipecolylxylidine in humans is mainly catalyzed by CYP3A. Drug Metab Dispos. 2000 Apr;28(4):383-5. | ||||
| 80 | DAILYMED.nlm.nih.gov: JEVTANA- cabazitaxel kit. | ||||
| 81 | The effect of CYP2C19 and CYP2D6 genotypes on the metabolism of clomipramine in Japanese psychiatric patients. J Clin Psychopharmacol. 2001 Dec;21(6):549-55. | ||||
| 82 | The biotransformation of clomipramine in vitro, identification of the cytochrome P450s responsible for the separate metabolic pathways. J Pharmacol Exp Ther. 1996 Jun;277(3):1659-64. | ||||
| 83 | Colchicine down-regulates cytochrome P450 2B6, 2C8, 2C9, and 3A4 in human hepatocytes by affecting their glucocorticoid receptor-mediated regulation. Mol Pharmacol. 2003 Jul;64(1):160-9. | ||||
| 84 | Conjugated oestrogens/bazedoxifene. Aust Prescr. 2017 Jun;40(3):114-115. | ||||
| 85 | Identification of human liver cytochrome P450 isoforms involved in the in vitro metabolism of cyclobenzaprine. Drug Metab Dispos. 1996 Jul;24(7):786-91. | ||||
| 86 | The effect of new lipophilic chelators on the activities of cytosolic reductases and P450 cytochromes involved in the metabolism of anthracycline antibiotics: studies in vitro. Physiol Res. 2004;53(6):683-91. | ||||
| 87 | Interaction of disulfiram with antiretroviral medications: efavirenz increases while atazanavir decreases disulfiram effect on enzymes of alcohol metabolism. Am J Addict. 2014 Mar-Apr;23(2):137-44. | ||||
| 88 | Pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet. 2015 Oct;54(10):1057-69. | ||||
| 89 | [Enzalutamide in castration resistant prostate cancer.] | ||||
| 90 | Microsomal catalyzed N-hydroxylation of guanfacine and reduction of N-hydroxyguanfacine. Arch Pharm (Weinheim). 1997 Oct;330(9-10):303-6. | ||||
| 91 | Metabolism of anabolic steroids by recombinant human cytochrome P450 enzymes. Gas chromatographic-mass spectrometric determination of metabolites. J Chromatogr B Biomed Sci Appl. 1999 Nov 26;735(1):73-83. | ||||
| 92 | Role of cytochrome p450 isoenzymes 3A and 2D6 in the in vivo metabolism of mirabegron, a beta-3-adrenoceptor agonist. Clin Drug Investig. 2013 Jun;33(6):429-40. | ||||
| 93 | Identification of the human cytochrome P450 enzymes involved in the in vitro biotransformation of lynestrenol and norethindrone. J Steroid Biochem Mol Biol. 2008 May;110(1-2):56-66. | ||||
| 94 | Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015 Jul;27(2):115-45. | ||||
| 95 | Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010 Jul;70(1):78-87. | ||||
| 96 | Pioglitazone is metabolised by CYP2C8 and CYP3A4 in vitro: potential for interactions with CYP2C8 inhibitors. Basic Clin Pharmacol Toxicol. 2006 Jul;99(1):44-51. | ||||
| 97 | In vitro comparative inhibition profiles of major human drug metabolising cytochrome P450 isozymes (CYP2C9, CYP2D6 and CYP3A4) by HMG-CoA reductase inhibitors. Eur J Clin Pharmacol. 1996;50(3):209-15. | ||||
| 98 | Ranolazine: a contemporary review. J Am Heart Assoc. 2016 Mar 15;5(3):e003196. | ||||
| 99 | Comparative efficacy and safety of the novel oral anticoagulants dabigatran, rivaroxaban and apixaban in preclinical and clinical development. Thromb Haemost. 2010 Mar;103(3):572-85. | ||||
| 100 | Dose-dependent induction of cytochrome P450 (CYP) 3A4 and activation of pregnane X receptor by topiramate. Epilepsia. 2003 Dec;44(12):1521-8. | ||||
| 101 | In vitro characterization of the human biotransformation and CYP reaction phenotype of ET-743 (Yondelis, Trabectidin), a novel marine anti-cancer drug. Invest New Drugs. 2006 Jan;24(1):3-14. | ||||
| 102 | Comparison of 19F NMR and 14C measurements for the assessment of ADME of BYL719 (Alpelisib) in humans. Drug Metab Dispos. 2017 Aug;45(8):900-907. | ||||
| 103 | The pharmacology and clinical pharmacokinetics of apomorphine SL BJU Int. 2001 Oct;88 Suppl 3:18-21. doi: 10.1046/j.1464-4096.2001.00124.x. | ||||
| 104 | Metabolism of beclomethasone dipropionate by cytochrome P450 3A enzymes. J Pharmacol Exp Ther. 2013 May;345(2):308-16. | ||||
| 105 | Comparative kinetics of metabolism of beclomethasone propionate esters in human lung homogenates and plasma | ||||
| 106 | Identification of human hepatic cytochrome p450 enzymes involved in the biotransformation of cholic and chenodeoxycholic acid. Drug Metab Dispos. 2008 Oct;36(10):1983-91. | ||||
| 107 | Genetic polymorphism of cytochrome P450 2D6 determines oestrogen receptor activity of the major infertility drug clomiphene via its active metabolites | ||||
| 108 | FDA label of Copanlisib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 109 | Mechanisms and clinical significance of pharmacokinetic-based drug-drug interactions with drugs approved by the U.S. Food and Drug Administration in 2017. Drug Metab Dispos. 2019 Feb;47(2):135-144. | ||||
| 110 | CYP3A Specifically Catalyzes 1-Hydroxylation of Deoxycholic Acid: Characterization and Enzymatic Synthesis of a Potential Novel Urinary Biomarker for CYP3A Activity | ||||
| 111 | Edoxaban drug-drug interactions with ketoconazole, erythromycin, and cyclosporine. Br J Clin Pharmacol. 2016 Dec;82(6):1591-1600. | ||||
| 112 | The role of CYP2C and CYP3A in the disposition of 3-keto-desogestrel after administration of desogestrel. Br J Clin Pharmacol. 2005 Jul;60(1):69-75. | ||||
| 113 | Human reductive halothane metabolism in vitro is catalyzed by cytochrome P450 2A6 and 3A4. Drug Metab Dispos. 1996 Sep;24(9):976-83. | ||||
| 114 | The effect of CYP2D6 drug-drug interactions on hydrocodone effectiveness. Acad Emerg Med. 2014 Aug;21(8):879-85. | ||||
| 115 | Regulation of the CYP3A4 gene by hydrocortisone and xenobiotics: role of the glucocorticoid and pregnane X receptors. Drug Metab Dispos. 2000 May;28(5):493-6. | ||||
| 116 | Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2002 Jul;30(7):853-8. | ||||
| 117 | FDA label of Levocetirizine dihydrochloride. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 118 | Regulation of drug-metabolizing cytochrome P450 enzymes by glucocorticoids. Drug Metab Rev. 2010 Nov;42(4):621-35. | ||||
| 119 | Is meperidine the drug that just won't die? J Pediatr Pharmacol Ther. 2011 Jul;16(3):167-9. | ||||
| 120 | Cytochrome P450 enzyme regulation by glucocorticoids and consequences in terms of drug interaction. Expert Opin Drug Metab Toxicol. 2014 Mar;10(3):425-35. | ||||
| 121 | Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood. 2011 Feb 24;117(8):e75-87. | ||||
| 122 | FDA Approved Drug Products: TAGRISSO (osimertinib) tablets for oral use | ||||
| 123 | The Influence of CYP3A4 Genetic Polymorphism and Proton Pump Inhibitors on Osimertinib Metabolism | ||||
| 124 | Biocatalytic synthesis and structure elucidation of cyclized metabolites of the deacetylase inhibitor panobinostat (LBH589) Drug Metab Dispos. 2012 May;40(5):1041-50. doi: 10.1124/dmd.111.043620. | ||||
| 125 | The clinical pharmacology profile of the new antiepileptic drug perampanel: A novel noncompetitive AMPA receptor antagonist. Epilepsia. 2015 Jan;56(1):12-27. | ||||
| 126 | Mass balance, pharmacokinetics and metabolism of the selective S1P1 receptor modulator ponesimod in humans | ||||
| 127 | DrugBank(Pharmacology-Metabolism):Ponesimod | ||||
| 128 | Quinine induced simvastatin toxicity through cytochrome inhibition - a case report. BMC Geriatr. 2016 Oct 1;16(1):168. | ||||
| 129 | FDA Label of Regorafenib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 130 | Method development and validation for simultaneous determination of six tyrosine kinase inhibitors and two active metabolites in human plasma/serum using UPLC-MS/MS for therapeutic drug monitoring | ||||
| 131 | Viral target and metabolism-based rationale for combined use of recently authorized small molecule COVID-19 medicines: Molnupiravir, nirmatrelvir, and remdesivir | ||||
| 132 | Identification of cytochrome P450 isozymes involved in metabolism of the alpha1-adrenoceptor blocker tamsulosin in human liver microsomes. Xenobiotica. 1998 Oct;28(10):909-22. | ||||
| 133 | Physiologically based pharmacokinetic (PBPK) modelling of tamsulosin related to CYP2D6*10 allele | ||||
| 134 | Drug interactions during therapy with three major groups of antimicrobial agents. Expert Opin Pharmacother. 2006 Apr;7(6):639-51. | ||||
| 135 | Screening for inhibitory effects of antineoplastic agents on CYP3A4 in human liver microsomes. Int J Clin Pharmacol Ther. 2001 Dec;39(12):517-28. | ||||
| 136 | Open-label, single-center, phase I trial to investigate the mass balance and absolute bioavailability of the highly selective oral MET inhibitor tepotinib in healthy volunteers | ||||
| 137 | Identification of Iminium Intermediates Generation in the Metabolism of Tepotinib Using LC-MS/MS: In Silico and Practical Approaches to Bioactivation Pathway Elucidation | ||||
| 138 | Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010 Sep;38(9):1514-21. | ||||
| 139 | Elimination of tucatinib, a small molecule kinase inhibitor of HER2, is primarily governed by CYP2C8 enantioselective oxidation of gem-dimethyl | ||||
| 140 | Pharmacogenetic determinants of human liver microsomal alfentanil metabolism and the role of cytochrome P450 3A5. Anesthesiology. 2005 Mar;102(3):550-6. | ||||
| 141 | Clinical pharmacokinetics and pharmacodynamics of aliskiren. Clin Pharmacokinet. 2008;47(8):515-31. | ||||
| 142 | DailyMed : Alitretinoin | ||||
| 143 | Metabolism of beta-arteether to dihydroqinghaosu by human liver microsomes and recombinant cytochrome P450 | ||||
| 144 | Clinical pharmacology of axitinib. Clin Pharmacokinet. 2013 Sep;52(9):713-25. | ||||
| 145 | Identification of the human and animal hepatic cytochromes P450 involved in clonazepam metabolism. Fundam Clin Pharmacol. 1993;7(2):69-75. | ||||
| 146 | Brexpiprazole: first global approval. Drugs. 2015 Sep;75(14):1687-97. | ||||
| 147 | Effect of cytochrome P450 (CYP) inducers on caffeine metabolism in the rat. Pharmacol Rep. 2007 May-Jun;59(3):296-305. | ||||
| 148 | Role of cytochrome P450 2D6 genetic polymorphism in carvedilol hydroxylation in vitro. Drug Des Devel Ther. 2016 Jun 8;10:1909-16. | ||||
| 149 | Cisapride: a potential model substrate to assess cytochrome P4503A4 activity in vivo. Clin Pharmacol Ther. 2003 Mar;73(3):209-22. | ||||
| 150 | Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities? Drug Metab Dispos. 2001 Nov;29(11):1514-20. | ||||
| 151 | Comparative study of the metabolism of drug substrates by human cytochrome P450 3A4 expressed in bacterial, yeast and human lymphoblastoid cells. Xenobiotica. 2002 Nov;32(11):937-47. | ||||
| 152 | Comparative analysis of human CYP3A4 and rat CYP3A1 induction and relevant gene expression by bisphenol A and diethylstilbestrol: implications for toxicity testing paradigms. Reprod Toxicol. 2013 Jun;37:24-30. | ||||
| 153 | Species differences in the toxicity and cytochrome P450 IIIA-dependent metabolism of digitoxin. Mol Pharmacol. 1991 Nov;40(5):859-67. | ||||
| 154 | Pharmacogenomics as molecular autopsy for forensic toxicology: genotyping cytochrome P450 3A4*1B and 3A5*3 for 25 fentanyl cases J Anal Toxicol. 2005 Oct;29(7):590-8. doi: 10.1093/jat/29.7.590. | ||||
| 155 | The N-demethylation of the doxepin isomers is mainly catalyzed by the polymorphic CYP2C19. Pharm Res. 2002 Jul;19(7):1034-7. | ||||
| 156 | Role of CYP3A in oral contraceptives clearance. Clin Transl Sci. 2018 May;11(3):251-260. | ||||
| 157 | CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J Bone Miner Res. 2004 Apr;19(4):680-8. | ||||
| 158 | Effect of dexamethasone on cytochrome P-450 mediated metabolism of 2-acetylaminofluorene in cultured rat hepatocytes. Biochem Pharmacol. 1987 Jan 15;36(2):237-43. | ||||
| 159 | Identification of a novel glutathione conjugate of flutamide in incubations with human liver microsomes. Drug Metab Dispos. 2007 Jul;35(7):1081-8. | ||||
| 160 | ClinicalTrials.gov (NCT02456883) Study to Investigate the Absorption, Metabolism and Excretion of [14C] ASP2215 in Patients With Advanced Solid Tumors. | ||||
| 161 | Itraconazole-Induced Increases in Gilteritinib Exposure Are Mediated by CYP3A and OATP1B | ||||
| 162 | Assessment of the contributions of CYP3A4 and CYP3A5 in the metabolism of the antipsychotic agent haloperidol to its potentially neurotoxic pyridinium metabolite and effect of antidepressants on the bioactivation pathway. Drug Metab Dispos. 2003 Mar;31(3):243-9. | ||||
| 163 | An evaluation of the cytochrome P450 induction potential of pantoprazole in primary human hepatocytes. Chem Biol Interact. 1998 Jul 3;114(1-2):1-13. | ||||
| 164 | Disposition of lasofoxifene, a next-generation selective estrogen receptor modulator, in healthy male subjects | ||||
| 165 | DrugBank(Pharmacology-Metabolism):Lasofoxifene | ||||
| 166 | Influence of CYP3A4/5 polymorphisms in the pharmacokinetics of levonorgestrel: a pilot study. Biomedica. 2012 Oct-Dec;32(4):570-7. | ||||
| 167 | In?vitro assessment of the potential for dolutegravir to affect hepatic clearance of levonorgestrel | ||||
| 168 | Identification of human cytochrome P450 and flavin-containing monooxygenase enzymes involved in the metabolism of lorcaserin, a novel selective human 5-hydroxytryptamine 2C agonist. Drug Metab Dispos. 2012 Apr;40(4):761-71. | ||||
| 169 | Investigation of the effects of ketoconazole on the pharmacokinetics of macitentan, a novel dual endothelin receptor antagonist, in healthy subjects. Clin Pharmacokinet. 2013 Aug;52(8):685-92. | ||||
| 170 | Drug-drug and food-drug pharmacokinetic interactions with new insulinotropic agents repaglinide and nateglinide. Clin Pharmacokinet. 2007;46(2):93-108. | ||||
| 171 | Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999 Dec;27(12):1488-95. | ||||
| 172 | Extent of radiosensitization by the PARP inhibitor olaparib depends on its dose, the radiation dose and the integrity of the homologous recombination pathway of tumor cells. Radiother Oncol. 2015 Sep;116(3):358-65. | ||||
| 173 | The effect of rifampin on the pharmacokinetics of oral and intravenous ondansetron. Clin Pharmacol Ther. 1999 Apr;65(4):377-81. | ||||
| 174 | Pomalidomide: evaluation of cytochrome P450 and transporter-mediated drug-drug interaction potential in vitro and in healthy subjects. J Clin Pharmacol. 2015 Feb;55(2):168-78. | ||||
| 175 | Effect of selective serotonin reuptake inhibitors on the oxidative metabolism of propafenone: in vitro studies using human liver microsomes. J Clin Psychopharmacol. 2000 Aug;20(4):428-34. | ||||
| 176 | Cytochrome P450 isozymes involved in propranolol metabolism in human liver microsomes. The role of CYP2D6 as ring-hydroxylase and CYP1A2 as N-desisopropylase. Drug Metab Dispos. 1994 Nov-Dec;22(6):909-15. | ||||
| 177 | Tolerance and pharmacokinetic interactions of rifabutin and clarithromycin in human immunodeficiency virus-infected volunteers | ||||
| 178 | Receptor-binding and pharmacokinetic properties of dopaminergic agonists. Curr Top Med Chem. 2008;8(12):1049-67. | ||||
| 179 | PubChem:SR141716A | ||||
| 180 | Brain accumulation of tivozanib is restricted by ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein) in mice | ||||
| 181 | Effect of the Catechol-O-Methyltransferase Inhibitors Tolcapone and Entacapone on Fatty Acid Metabolism in HepaRG Cells | ||||
| 182 | Involvement of cytochrome P450 3A enzyme family in the major metabolic pathways of toremifene in human liver microsomes. Biochem Pharmacol. 1994 May 18;47(10):1883-95. | ||||
| 183 | Metabolic activation of the nontricyclic antidepressant trazodone to electrophilic quinone-imine and epoxide intermediates in human liver microsomes and recombinant P4503A4. Chem Biol Interact. 2005 Jun 30;155(1-2):10-20. | ||||
| 184 | The effect of ketoconazole on the pharmacokinetics and pharmacodynamics of inhaled fluticasone furoate and vilanterol trifenatate in healthy subjects. Br J Clin Pharmacol. 2013 Jun;75(6):1478-87. | ||||
| 185 | Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr Drug Metab. 2008 Jun;9(5):410-8. | ||||
| 186 | Bioactivation of the tricyclic antidepressant amitriptyline and its metabolite nortriptyline to arene oxide intermediates in human liver microsomes and recombinant P450s. Chem Biol Interact. 2008 May 9;173(1):59-67. | ||||
| 187 | Differential inhibition of cytochrome P450 3A4, 3A5 and 3A7 by five human immunodeficiency virus (HIV) protease inhibitors in vitro. Basic Clin Pharmacol Toxicol. 2006 Jan;98(1):79-85. | ||||
| 188 | The effect of grapefruit juice on the time-dependent decline of artemether plasma levels in healthy subjects. Clin Pharmacol Ther. 1999 Oct;66(4):408-14. | ||||
| 189 | An update on pharmacological treatment of erectile dysfunction with phosphodiesterase type 5 inhibitors. Expert Opin Pharmacother. 2013 Jul;14(10):1333-44. | ||||
| 190 | FDA label of Baricitinib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 191 | DrugBank(Pharmacology-Metabolism):Baricitinib | ||||
| 192 | Glucuronidation by UGT1A1 is the dominant pathway of the metabolic disposition of belinostat in liver cancer patients. PLoS One. 2013;8(1):e54522. | ||||
| 193 | DrugBank(Pharmacology-Metabolism):Betrixaban | ||||
| 194 | Brigatinib: First Global Approval Drugs. 2017 Jul;77(10):1131-1135. doi: 10.1007/s40265-017-0776-3. | ||||
| 195 | Metabolite Profiling and Reaction Phenotyping for the in Vitro Assessment of the Bioactivation of Bromfenac ? Chem Res Toxicol. 2020 Jan 21;33(1):249-257. doi: 10.1021/acs.chemrestox.9b00268. | ||||
| 196 | Effects of cytochrome P450 (CYP) 3A4 inhibitors on the anxiolytic action of tandospirone in rat contextual conditioned fear. Prog Neuropsychopharmacol Biol Psychiatry. 2007 May 9;31(4):926-31. | ||||
| 197 | Carbamazepine pharmacokinetics are not affected by zonisamide: in vitro mechanistic study and in vivo clinical study in epileptic patients. Epilepsy Res. 2004 Nov;62(1):1-11. | ||||
| 198 | FDA label of Cenobamate. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 199 | Pharmacokinetics and metabolism of the novel muscarinic receptor agonist SNI-2011 in rats and dogs. Arzneimittelforschung. 2003;53(1):26-33. | ||||
| 200 | Clinical pharmacokinetic and pharmacodynamic profile of cinacalcet hydrochloride. Clin Pharmacokinet. 2009;48(5):303-11. | ||||
| 201 | In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: importance of CYP2C19. Drug Metab Dispos. 2004 Nov;32(11):1279-86. | ||||
| 202 | Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004 Nov;32(11):1201-8. | ||||
| 203 | Prediction of tacrolimus metabolism and dosage requirements based on CYP3A4 phenotype and CYP3A5(*)3 genotype in Chinese renal transplant recipients. Acta Pharmacol Sin. 2016 Apr;37(4):555-60. | ||||
| 204 | Effects of ketoconazole on cyclophosphamide metabolism: evaluation of CYP3A4 inhibition effect using the in vitro and in vivo models. Exp Anim. 2018 Feb 9;67(1):71-82. | ||||
| 205 | Physiologically based pharmacokinetic modeling to identify physiological and molecular characteristics driving variability in drug exposure. Clin Pharmacol Ther. 2018 Dec;104(6):1219-1228. | ||||
| 206 | The clinical pharmacokinetics of darifenacin. Clin Pharmacokinet. 2006;45(4):325-50. | ||||
| 207 | Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009 Dec;35(8):692-706. | ||||
| 208 | Desvenlafaxine succinate for major depressive disorder | ||||
| 209 | Influence of CYP2D6 genotype on the disposition of the enantiomers of venlafaxine and its major metabolites in postmortem femoral blood | ||||
| 210 | Desvenlafaxine in the treatment of major depressive disorder. Expert Opin Pharmacother. 2011 Dec;12(18):2923-8. | ||||
| 211 | Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos. 2002 Aug;30(8):883-91. | ||||
| 212 | Mechanisms of action, pharmacology and interactions of dolutegravir. Enferm Infecc Microbiol Clin. 2015 Mar;33 Suppl 1:2-8. | ||||
| 213 | Donepezil plasma concentrations, CYP2D6 and CYP3A4 phenotypes, and cognitive outcome in Alzheimer's disease. Eur J Clin Pharmacol. 2016 Jun;72(6):711-7. | ||||
| 214 | Expression levels and activation of a PXR variant are directly related to drug resistance in osteosarcoma cell lines. Cancer. 2007 Mar 1;109(5):957-65. | ||||
| 215 | Drug safety evaluation of dronedarone in atrial fibrillation. Expert Opin Drug Saf. 2012 Nov;11(6):1023-45. | ||||
| 216 | Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004 Nov;44(11):1273-81. | ||||
| 217 | FDA label of Erdafitinib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 218 | Evidence for the Metabolic Activation of Deferasirox In Vitro and In Vivo | ||||
| 219 | A mechanistic approach to antiepileptic drug interactions. Ann Pharmacother. 1998 May;32(5):554-63. | ||||
| 220 | O-Dealkylation of fluoxetine in relation to CYP2C19 gene dose and involvement of CYP3A4 in human liver microsomes. J Pharmacol Exp Ther. 2002 Jan;300(1):105-11. | ||||
| 221 | Is 1-aminobenzotriazole an appropriate in vitro tool as a nonspecific cytochrome P450 inactivator? Drug Metab Dispos. 2009 Jan;37(1):10-3. | ||||
| 222 | Effects of fluvastatin on the pharmacokinetics of eepaglinide: possible role of CYP3A4 and P-glycoprotein inhibition by fluvastatin. Korean J Physiol Pharmacol. 2013 Jun;17(3):245-51. | ||||
| 223 | Atazanavir for the treatment of human immunodeficiency virus infection. Pharmacotherapy. 2004 Dec;24(12):1732-47. | ||||
| 224 | PharmGKB summary: ibuprofen pathways. Pharmacogenet Genomics. 2015 Feb;25(2):96-106. | ||||
| 225 | Variability in metabolism of imipramine and desipramine using urinary excretion data. J Anal Toxicol. 2014 Jul-Aug;38(6):368-74. | ||||
| 226 | Infigratinib Is a Reversible Inhibitor and Mechanism-Based Inactivator of Cytochrome P450 3A4 | ||||
| 227 | Identification of Infigratinib as a Potent Reversible Inhibitor and Mechanism-Based Inactivator of CYP2J2: Nascent Evidence for a Potential In Vivo Metabolic Drug-Drug Interaction with Rivaroxaban | ||||
| 228 | The effect of isotretinoin on the pharmacokinetics and pharmacodynamics of ethinyl estradiol and norethindrone. Clin Pharmacol Ther. 2004 May;75(5):464-75. | ||||
| 229 | Human cytochrome P450s involved in the metabolism of 9-cis- and 13-cis-retinoic acids | ||||
| 230 | Isocratic high-performance liquid chromatographic method for the separation of isradipine and its main metabolites. Application to in vitro metabolization by h3A4/OR cells J Chromatogr B Biomed Sci Appl. 1997 Jun 6;693(2):359-66. doi: 10.1016/s0378-4347(97)00048-0. | ||||
| 231 | Effects of rifampin on the pharmacokinetics of a single dose of istradefylline in healthy subjects. J Clin Pharmacol. 2018 Feb;58(2):193-201. | ||||
| 232 | Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes. Xenobiotica. 1998 Mar;28(3):313-21. | ||||
| 233 | Pharmacokinetics, absorption, metabolism, and excretion of [(14)C]ivosidenib (AG-120) in healthy male subjects | ||||
| 234 | Preclinical Drug Metabolism, Pharmacokinetic, and Pharmacodynamic Profiles of Ivosidenib, an Inhibitor of Mutant Isocitrate Dehydrogenase 1 for Treatment of Isocitrate Dehydrogenase 1-Mutant Malignancies | ||||
| 235 | Prediction of drug-induced liver injury in HepG2 cells cultured with human liver microsomes. Chem Res Toxicol. 2015 May 18;28(5):872-85. | ||||
| 236 | In vitro metabolism studies on the isoxazole ring scission in the anti-inflammatory agent lefluonomide to its active alpha-cyanoenol metabolite A771726: mechanistic similarities with the cytochrome P450-catalyzed dehydration of aldoximes Drug Metab Dispos. 2003 Oct;31(10):1240-50. doi: 10.1124/dmd.31.10.1240. | ||||
| 237 | Characterization of Stereoselective Metabolism, Inhibitory Effect on Uric Acid Uptake Transporters, and Pharmacokinetics of Lesinurad Atropisomers Drug Metab Dispos. 2019 Feb;47(2):104-113. doi: 10.1124/dmd.118.080549. | ||||
| 238 | Absorption, Metabolism, Distribution, and Excretion of Letermovir Curr Drug Metab. 2021;22(10):784-794. doi: 10.2174/1389200222666210223112826. | ||||
| 239 | Resin haemoperfusion in levomepromazine poisoning: evaluation of effect on plasma drug and metabolite levels | ||||
| 240 | Pharmacokinetics and pharmacodynamics of lurasidone hydrochloride, a second-generation antipsychotic: a systematic review of the published literature. Clin Pharmacokinet. 2017 May;56(5):493-503. | ||||
| 241 | Chemical stability-indicating HPLC study of fixed-dosage combination containing metoprolol tartrate and hydrochlorothiazide. | ||||
| 242 | Cytochromes P450: a structure-based summary of biotransformations using representative substrates Drug Metab Rev. 2008;40(1):1-100. doi: 10.1080/03602530802309742. | ||||
| 243 | Selection of alternative CYP3A4 probe substrates for clinical drug interaction studies using in vitro data and in vivo simulation. Drug Metab Dispos. 2010 Jun;38(6):981-7. | ||||
| 244 | Modulation of UDP-glucuronosyltransferase 2B7 function by cytochrome P450s in vitro: differential effects of CYP1A2, CYP2C9 and CYP3A4. Biol Pharm Bull. 2005 Oct;28(10):2026-7. | ||||
| 245 | Nefazodone, meta-chlorophenylpiperazine, and their metabolites in vitro: cytochromes mediating transformation, and P450-3A4 inhibitory actions. Psychopharmacology (Berl). 1999 Jul;145(1):113-22. | ||||
| 246 | Pharmacodynamics, pharmacokinetics and clinical efficacy of neratinib in HER2-positive breast cancer and breast cancer with HER2 mutations. Expert Opin Drug Metab Toxicol. 2016 Aug;12(8):947-57. | ||||
| 247 | FDA label of Pexidartinib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 248 | Coadministration of lopinavir/ritonavir and phenytoin results in two-way drug interaction through cytochrome P-450 induction. J Acquir Immune Defic Syndr. 2004 Aug 15;36(5):1034-40. | ||||
| 249 | Metabolic profile of oxazepam and related benzodiazepines: clinical and forensic aspects. Drug Metab Rev. 2017 Nov;49(4):451-463. | ||||
| 250 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development Curr Med Chem. 2009;16(27):3480-675. doi: 10.2174/092986709789057635. | ||||
| 251 | DrugBank(Pharmacology-Metabolism):Propoxyphene Hydrochloride | ||||
| 252 | 10th North American ISSX meeting. October 24-28, 2000. Indianapolis, Indiana, USA. Abstracts. Drug Metab Rev. 2000;32 Suppl 2:137-340. | ||||
| 253 | Potential of the novel antiretroviral drug rilpivirine to modulate the expression and function of drug transporters and drug-metabolising enzymes in vitro. Int J Antimicrob Agents. 2013 May;41(5):484-7. | ||||
| 254 | Metabolism of a new local anesthetic, ropivacaine, by human hepatic cytochrome P450. Anesthesiology. 1995 Jan;82(1):214-20. | ||||
| 255 | Evaluation of absorption, distribution, metabolism, and excretion of [(14)C]-rucaparib, a poly(ADP-ribose) polymerase inhibitor, in patients with advanced solid tumors Invest New Drugs. 2020 Jun;38(3):765-775. doi: 10.1007/s10637-019-00815-2. | ||||
| 256 | A comparison of the effects of 3-hydroxy-3-methylglutaryl-coenzyme a (HMG-CoA) reductase inhibitors on the CYP3A4-dependent oxidation of mexazolam in vitro. Drug Metab Dispos. 2001 Mar;29(3):282-8. | ||||
| 257 | DrugBank(Pharmacology-Metabolism):Simvastatin | ||||
| 258 | CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab Dispos. 2002 Jul;30(7):795-804. | ||||
| 259 | Suvorexant: a promising, novel treatment for insomnia. Neuropsychiatr Dis Treat. 2016 Feb 25;12:491-5. | ||||
| 260 | The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a janus kinase inhibitor, in humans. Drug Metab Dispos. 2014 Apr;42(4):759-73. | ||||
| 261 | The dawn of hedgehog inhibitors: Vismodegib. J Pharmacol Pharmacother. 2013 Jan;4(1):4-7. | ||||
| 262 | FDA label of Zanubrutinib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 263 | In vitro investigations into the roles of CYP450 enzymes and drug transporters in the drug interactions of zanubrutinib, a covalent Bruton's tyrosine kinase inhibitor | ||||
| 264 | Zolpidem pharmacokinetics and pharmacodynamics in metabolic interactions involving CYP3A: sex as a differentiating factor. Eur J Clin Pharmacol. 2010 Sep;66(9):955. | ||||
| 265 | In vitro metabolism of alectinib, a novel potent ALK inhibitor, in human: contribution of CYP3A enzymes. Xenobiotica. 2018 Jun;48(6):546-554. | ||||
| 266 | In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br J Clin Pharmacol. 2010 Dec;70(6):854-69. | ||||
| 267 | Assessment of the potential pharmacokinetic and pharmacodynamic interactions between erythromycin and argatroban. J Clin Pharmacol. 1999 May;39(5):513-9. | ||||
| 268 | In vitro identification of the human cytochrome P-450 enzymes involved in the N-demethylation of azelastine. Drug Metab Dispos. 1999 Aug;27(8):942-6. | ||||
| 269 | Bedaquiline metabolism: enzymes and novel metabolites. Drug Metab Dispos. 2014 May;42(5):863-6. | ||||
| 270 | Studies on Drug Interactions between CYP3A4 Inhibitors and Glucocorticoids. | ||||
| 271 | No relevant effect of ursodeoxycholic acid on cytochrome P450 3A metabolism in primary biliary cirrhosis. Hepatology. 2005 Mar;41(3):595-602. | ||||
| 272 | Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010 Jan-Feb;19(1):4-16. | ||||
| 273 | Practical management of adverse events associated with cabozantinib treatment in patients with renal-cell carcinoma. Onco Targets Ther. 2017 Oct 19;10:5053-5064. | ||||
| 274 | Effect of classic and atypical neuroleptics on cytochrome P450 3A (CYP3A) in rat liver. Pharmacol Rep. 2012;64(6):1411-8. | ||||
| 275 | PharmGKB summary: citalopram pharmacokinetics pathway. Pharmacogenet Genomics. 2011 Nov;21(11):769-72. | ||||
| 276 | Pharmacokinetic variability of clarithromycin and differences in CYP3A4 activity in patients with cystic fibrosis. J Cyst Fibros. 2014 Mar;13(2):179-85. | ||||
| 277 | CYP2D6 mediates 4-hydroxylation of clonidine in vitro: implication for pregnancy-induced changes in clonidine clearance. Drug Metab Dispos. 2010 Sep;38(9):1393-6. | ||||
| 278 | Drug metabolism and atypical antipsychotics. Eur Neuropsychopharmacol. 1999 Jun;9(4):301-9. | ||||
| 279 | Pharmacokinetics and metabolism of delamanid, a novel anti-tuberculosis drug, in animals and humans: importance of albumin metabolism in vivo. Drug Metab Dispos. 2015 Aug;43(8):1267-76. | ||||
| 280 | Significance of metabolism in the disposition and action of the antidysrhythmic drug, dofetilide. In vitro studies and correlation with in vivo data. Drug Metab Dispos. 1996 Apr;24(4):447-55. | ||||
| 281 | Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of dolasetron. Comparison with other indole-containing 5-HT3 antagonists. Drug Metab Dispos. 1996 May;24(5):602-9. | ||||
| 282 | Population pharmacokinetics of doxapram in low-birth-weight Japanese infants with apnea. Eur J Pediatr. 2015 Apr;174(4):509-18. | ||||
| 283 | Entrectinib: first global approval. Drugs. 2019 Sep;79(13):1477-1483. | ||||
| 284 | Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of ethosuximide by human hepatic microsomal enzymes. Xenobiotica. 2003 Mar;33(3):265-76. | ||||
| 285 | Pharmacokinetic interactions between etravirine and non-antiretroviral drugs. Clin Pharmacokinet. 2011 Jan;50(1):25-39. | ||||
| 286 | The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S. | ||||
| 287 | In vitro metabolism of exemestane by hepatic cytochrome P450s: impact of nonsynonymous polymorphisms on formation of the active metabolite 17beta-dihydroexemestane. Pharmacol Res Perspect. 2017 Apr 27;5(3):e00314. | ||||
| 288 | Evidence that famciclovir (BRL 42810) and its associated metabolites do not inhibit the 6 beta-hydroxylation of testosterone in human liver microsomes. Drug Metab Dispos. 1993 Jan-Feb;21(1):18-23. | ||||
| 289 | Three-dimensional-quantitative structure activity relationship analysis of cytochrome P-450 3A4 substrates. J Pharmacol Exp Ther. 1999 Oct;291(1):424-33. | ||||
| 290 | LABEL:INGOLIMOD capsule | ||||
| 291 | Metabolic pathways of inhaled glucocorticoids by the CYP3A enzymes. Drug Metab Dispos. 2013 Feb;41(2):379-89. | ||||
| 292 | Basic review of the cytochrome p450 system. J Adv Pract Oncol. 2013 Jul;4(4):263-8. | ||||
| 293 | Effects of CYP3A4 inhibitors ketoconazole and verapamil and the CYP3A4 inducer rifampicin on the pharmacokinetic parameters of fostamatinib: results from In vitro and phase I clinical studies. Drugs R D. 2016 Mar;16(1):81-92. | ||||
| 294 | Clinical pharmacokinetics of fibric acid derivatives (fibrates). Clin Pharmacokinet. 1998 Feb;34(2):155-62. | ||||
| 295 | Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus. Pharmacoeconomics. 2004;22(6):389-411. | ||||
| 296 | Dehydrogenation of the indoline-containing drug 4-chloro-N-(2-methyl-1-indolinyl)-3-sulfamoylbenzamide (indapamide) by CYP3A4: correlation with in silico predictions. Drug Metab Dispos. 2009 Mar;37(3):672-84. | ||||
| 297 | Clinical pharmacokinetics and pharmacodynamics of nintedanib. Clin Pharmacokinet. 2019 Sep;58(9):1131-1147. | ||||
| 298 | Isoforms of cytochrome P450 on organic nitrate-derived nitric oxide release in human heart vessels. FEBS Lett. 1999 Jun 11;452(3):165-9. | ||||
| 299 | Ivabradine: new drug. Best avoided in stable angina. Prescrire Int. 2007 Apr;16(88):53-6. | ||||
| 300 | The effect of ketoconazole on the pharmacokinetics and pharmacodynamics of ixabepilone: a first in class epothilone B analogue in late-phase clinical development. Clin Cancer Res. 2008 May 1;14(9):2701-9. | ||||
| 301 | Clinical profile of levobupivacaine in regional anesthesia: A systematic review. J Anaesthesiol Clin Pharmacol. 2013 Oct;29(4):530-9. | ||||
| 302 | Exploration of catalytic properties of CYP2D6 and CYP3A4 through metabolic studies of levorphanol and levallorphan | ||||
| 303 | [Metabolic interaction between psychopharmaceuticals. Probable cause of exacerbation of hypothyroidism according to a case report]. Lakartidningen. 2002 Jun 20;99(25):2854-6. | ||||
| 304 | In vitro identification of the human cytochrome p450 enzymes involved in the oxidative metabolism of loxapine. Biopharm Drug Dispos. 2011 Oct;32(7):398-407. | ||||
| 305 | Metabolic Disposition of Lurbinectedin, a Potent Selective Inhibitor of Active Transcription of Protein-Coding Genes, in Nonclinical Species and Patients | ||||
| 306 | Evaluation of strategies for the assessment of drug-drug interactions involving cytochrome P450 enzymes. Eur J Drug Metab Pharmacokinet. 2018 Dec;43(6):737-750. | ||||
| 307 | A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001 Fall;7(3):249-64. | ||||
| 308 | Ema.europa:Nalmefene | ||||
| 309 | Naloxegol: treatment for opioid-induced constipation in chronic non-cancer pain. Ann Pharmacother. 2015 Mar;49(3):360-5. | ||||
| 310 | Ospemifene metabolism in humans in vitro and in vivo: metabolite identification, quantitation, and CYP assignment of major hydroxylations. Drug Metabol Drug Interact. 2013;28(3):153-61. | ||||
| 311 | Quantitative contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab Dispos. 2004 Apr;32(4):447-54. | ||||
| 312 | New orally active anticoagulant agents for the prevention and treatment of venous thromboembolism in cancer patients. Ther Clin Risk Manag. 2014 Jun 13;10:423-36. | ||||
| 313 | Pazopanib, a new therapy for metastatic soft tissue sarcoma. Expert Opin Pharmacother. 2013 May;14(7):929-35. | ||||
| 314 | Identification of the human cytochrome P450 isoforms mediating in vitro N-dealkylation of perphenazine. Br J Clin Pharmacol. 2000 Dec;50(6):563-71. | ||||
| 315 | Identification of cytochromes P450 2C9 and 3A4 as the major catalysts of phenprocoumon hydroxylation in vitro. Eur J Clin Pharmacol. 2004 May;60(3):173-82. | ||||
| 316 | Identification and characterization of human cytochrome P450 isoforms interacting with pimozide. J Pharmacol Exp Ther. 1998 May;285(2):428-37. | ||||
| 317 | Evaluation of the effect of multiple doses of rifampin on the pharmacokinetics and safety of ponatinib in healthy subjects. Clin Pharmacol Drug Dev. 2015 Sep;4(5):354-60. | ||||
| 318 | An overview of the role of selpercatinib and pralsetinib in RET-fusion-positive non-small cell lung cancer (NSCLC) | ||||
| 319 | Cytochrome P-450 2B6 is responsible for interindividual variability of propofol hydroxylation by human liver microsomes. Anesthesiology. 2001 Jan;94(1):110-9. | ||||
| 320 | Metabolism of the active metabolite of quetiapine, N-desalkylquetiapine in vitro. Drug Metab Dispos. 2012 Sep;40(9):1778-84. | ||||
| 321 | Effects of clarithromycin and verapamil on rabeprazole pharmacokinetics between CYP2C19 genotypes. Eur J Clin Pharmacol. 2006 Aug;62(8):597-603. | ||||
| 322 | The role of P-glycoprotein in the bioactivation of raloxifene. Drug Metab Dispos. 2006 Dec;34(12):2073-8. | ||||
| 323 | Effect of extended exposure to grapefruit juice on cytochrome P450 3A activity in humans: comparison with ritonavir. Clin Pharmacol Ther. 2006 Mar;79(3):243-54. | ||||
| 324 | In vitro identification of the P450 enzymes responsible for the metabolism of ropinirole. Drug Metab Dispos. 1997 Jul;25(7):840-4. | ||||
| 325 | Effect of saquinavir-ritonavir on cytochrome P450 3A4 activity in healthy volunteers using midazolam as a probe. Pharmacotherapy. 2009 Oct;29(10):1175-81. | ||||
| 326 | Clinical pharmacokinetics and pharmacodynamics of solifenacin. Clin Pharmacokinet. 2009;48(5):281-302. | ||||
| 327 | The effect of cimetidine on the formation of sulfamethoxazole hydroxylamine in patients with human immunodeficiency virus. J Clin Pharmacol. 1998 May;38(5):463-6. | ||||
| 328 | Identification of CYP3A4 as the primary cytochrome P450 responsible for the metabolism of tandospirone by human liver microsomes | ||||
| 329 | Clinical assessment of drug-drug interactions of tasimelteon, a novel dual melatonin receptor agonist. J Clin Pharmacol. 2015 Sep;55(9):1004-11. | ||||
| 330 | Managing drug-drug interactions with boceprevir and telaprevir. Clin Liver Dis (Hoboken). 2012 Apr 26;1(2):36-40. | ||||
| 331 | Characterization of human cytochromes P450 involved in theophylline 8-hydroxylation. Biochem Pharmacol. 1995 Jul 17;50(2):205-11. | ||||
| 332 | Extraction of structure-activity relationship information from high-throughput screening data Curr Med Chem. 2009;16(31):4049-57. doi: 10.2174/092986709789378189. | ||||
| 333 | Cytochrome P450 isozymes 3A4 and 2B6 are involved in the in vitro human metabolism of thiotepa to TEPA. Cancer Chemother Pharmacol. 2002 Jun;49(6):461-7. | ||||
| 334 | Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. | ||||
| 335 | In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes p450: role of cyp3a4 and cyp3a5. Drug Metab Dispos. 2003 Jul;31(7):938-44. | ||||
| 336 | Vemurafenib for the treatment of melanoma. Expert Opin Pharmacother. 2012 Dec;13(17):2533-43. | ||||
| 337 | Effect of disulfiram-mediated CYP2E1 inhibition on the disposition of vesnarinone | ||||
| 338 | Vilazodone HCl (Viibryd): A Serotonin Partial Agonist and Reuptake Inhibitor For the Treatment of Major Depressive Disorder. P T. 2012 Jan;37(1):28-31. | ||||
| 339 | Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos. 2012 Jul;40(7):1357-65. | ||||
| 340 | Fluconazole but not the CYP3A4 inhibitor, itraconazole, increases zafirlukast plasma concentrations. Eur J Clin Pharmacol. 2012 May;68(5):681-8. | ||||
| 341 | Metabolic interactions between acetaminophen (paracetamol) and two flavonoids, luteolin and quercetin, through in-vitro inhibition studies. J Pharm Pharmacol. 2017 Dec;69(12):1762-1772. | ||||
| 342 | Extended-release alfuzosin hydrochloride: a new alpha-adrenergic receptor antagonist for symptomatic benign prostatic hyperplasia. Am J Geriatr Pharmacother. 2004 Mar;2(1):14-23. | ||||
| 343 | No relevant interaction with alprazolam, caffeine, tolbutamide, and digoxin by treatment with a low-hyperforin St John's wort extract. Planta Med. 2005 Apr;71(4):331-7. | ||||
| 344 | In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Dispos. 2010 Mar;38(3):448-58. | ||||
| 345 | Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 2004;64(15):1715-36. | ||||
| 346 | FDA label:Avapritinib | ||||
| 347 | Avapritinib: First Approval | ||||
| 348 | Binimetinib Is a Potent Reversible and Time-Dependent Inhibitor of Cytochrome P450 1A2 Chem Res Toxicol. 2021 Apr 19;34(4):1169-1174. doi: 10.1021/acs.chemrestox.1c00036. | ||||
| 349 | Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1alpha,25-dihydroxyvitamin D(3): implications for drug-induced osteomalacia. Mol Pharmacol. 2006 Jan;69(1):56-65. | ||||
| 350 | Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. P T. 2015 Jul;40(7):451-62. | ||||
| 351 | DrugBank(Pharmacology-Metabolism):Chlordiazepoxide | ||||
| 352 | Identification of enzymes involved in phase I metabolism of ciclesonide by human liver microsomes. Eur J Drug Metab Pharmacokinet. 2005 Oct-Dec;30(4):275-86. | ||||
| 353 | Cytotoxicity, accumulation, and efflux of cisplatin and its metabolites in human ovarian carcinoma cells | ||||
| 354 | Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet. 2014 Jan;53(1):17-27. | ||||
| 355 | Drug interactions with new and investigational antiretrovirals. Clin Pharmacokinet. 2009;48(4):211-41. | ||||
| 356 | A long-standing mystery solved: the formation of 3-hydroxydesloratadine is catalyzed by CYP2C8 but prior glucuronidation of desloratadine by UDP-glucuronosyltransferase 2B10 is an obligatory requirement Drug Metab Dispos. 2015 Apr;43(4):523-33. doi: 10.1124/dmd.114.062620. | ||||
| 357 | Identification of drugs inhibiting the in vitro metabolism of tacrolimus by human liver microsomes. Br J Clin Pharmacol. 1996 Mar;41(3):187-90. | ||||
| 358 | Absorption, distribution, metabolism and excretion of [14C]dexlansoprazole in healthy male subjects. Clin Drug Investig. 2012 May 1;32(5):319-32. | ||||
| 359 | Omeprazole-associated digoxin toxicity. South Med J. 2007 Apr;100(4):400-2. | ||||
| 360 | Randomized pharmacokinetic and pharmacodynamic study of docetaxel: dosing based on body-surface area compared with individualized dosing based on cytochrome P450 activity estimated using a urinary metabolite of exogenous cortisol. J Clin Oncol. 2005 Feb 20;23(6):1061-9. | ||||
| 361 | LC-MS/MS determination of dutasteride and its major metabolites in human plasma | ||||
| 362 | Drug interactions between common dermatological medications and the oral anti-COVID-19 agents nirmatrelvir-ritonavir and molnupiravir | ||||
| 363 | Mass balance and drug interaction potential of intravenous eravacycline administered to healthy subjects. Antimicrob Agents Chemother. 2019 Feb 26;63(3). pii: e01810-18. | ||||
| 364 | Identification of human cytochrome P450 enzymes involved in the formation of 4-hydroxyestazolam from estazolam. Xenobiotica. 2005 May;35(5):455-65. | ||||
| 365 | Effects of morin on the pharmacokinetics of etoposide in rats. Biopharm Drug Dispos. 2007 Apr;28(3):151-6. | ||||
| 366 | Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44(5):467-94. | ||||
| 367 | FDA label of Fedratinib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 368 | Effects of the moderate CYP3A4 inhibitor, fluconazole, on the pharmacokinetics of fesoterodine in healthy subjects. Br J Clin Pharmacol. 2011 Aug;72(2):263-9. | ||||
| 369 | Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin Cancer Res. 2007 Jun 15;13(12):3731-7. | ||||
| 370 | PubChem:Grepafloxacin | ||||
| 371 | Influences of CYP2D6*10 polymorphisms on the pharmacokinetics of iloperidone and its metabolites in Chinese patients with schizophrenia: a population pharmacokinetic analysis. Acta Pharmacol Sin. 2016 Nov;37(11):1499-1508. | ||||
| 372 | Irinotecan and its active metabolite, SN-38: review of bioanalytical methods and recent update from clinical pharmacology perspectives. Biomed Chromatogr. 2010 Jan;24(1):104-23. | ||||
| 373 | Effects of itraconazole and tandospirone on the pharmacokinetics of perospirone. Ther Drug Monit. 2006 Feb;28(1):73-5. | ||||
| 374 | Clinical Pharmacokinetic and Pharmacodynamic Profile of Lenvatinib, an Orally Active, Small-Molecule, Multitargeted Tyrosine Kinase Inhibitor | ||||
| 375 | Paradoxical role of cytochrome P450 3A in the bioactivation and clinical effects of levo-alpha-acetylmethadol: importance of clinical investigations to validate in vitro drug metabolism studies. Clin Pharmacokinet. 2005;44(7):731-51. | ||||
| 376 | The Role of Levomilnacipran in the Management of Major Depressive Disorder: A Comprehensive Review Curr Neuropharmacol. 2016;14(2):191-9. doi: 10.2174/1570159x14666151117122458. | ||||
| 377 | Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7 Suppl 3:S11-8. | ||||
| 378 | Metabolic interactions with statins. Tidsskr Nor Laegeforen. 2001 Jan 20;121(2):189-93. | ||||
| 379 | DrugBank(Pharmacology-Metabolism):Macimorelin | ||||
| 380 | Metabolic profiling and cytochrome P450 reaction phenotyping of medroxyprogesterone acetate. Drug Metab Dispos. 2008 Nov;36(11):2292-8. | ||||
| 381 | Methadone metabolism and drug-drug interactions: in vitro and in vivo literature review. J Pharm Sci. 2018 Dec;107(12):2983-2991. | ||||
| 382 | Pharmacokinetic and pharmacodynamic interaction between mexiletine and propafenone in human beings. Clin Pharmacol Ther. 2000 Jul;68(1):44-57. | ||||
| 383 | Midostaurin, a novel protein kinase inhibitor for the treatment of acute myelogenous leukemia: insights from human absorption, metabolism, and excretion studies of a BDDCS II drug. Drug Metab Dispos. 2017 May;45(5):540-555. | ||||
| 384 | Pharmacogenomics knowledge for personalized medicine Clin Pharmacol Ther. 2012 Oct;92(4):414-7. doi: 10.1038/clpt.2012.96. | ||||
| 385 | Characterization of a phase 1 metabolite of mycophenolic acid produced by CYP3A4/5. Ther Drug Monit. 2004 Dec;26(6):600-8. | ||||
| 386 | DrugBank(Pharmacology-Metabolism):Mycophenolic acid | ||||
| 387 | Effects of olopatadine, a new antiallergic agent, on human liver microsomal cytochrome P450 activities. Drug Metab Dispos. 2002 Dec;30(12):1504-11. | ||||
| 388 | Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005 Jan;97(1):83-90. | ||||
| 389 | Drug-interaction between paclitaxel and goshajinkigan extract and its constituents | ||||
| 390 | Pharmacokinetics, metabolism and excretion of intravenous [l4C]-palonosetron in healthy human volunteers Biopharm Drug Dispos. 2004 Nov;25(8):329-37. doi: 10.1002/bdd.410. | ||||
| 391 | Interaction of proton pump inhibitors with cytochromes P450: consequences for drug interactions. Yale J Biol Med. 1996 May-Jun;69(3):203-9. | ||||
| 392 | An evaluation of potential mechanism-based inactivation of human drug metabolizing cytochromes P450 by monoamine oxidase inhibitors, including isoniazid. Br J Clin Pharmacol. 2006 May;61(5):570-84. | ||||
| 393 | Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Ther Drug Monit. 2007 Dec;29(6):687-710. | ||||
| 394 | Cytochrome P450 3A4-mediated oxidative conversion of a cyano to an amide group in the metabolism of pinacidil. Biochemistry. 2002 Feb 26;41(8):2712-8. | ||||
| 395 | Metabolic fate of pitavastatin, a new inhibitor of HMG-CoA reductase: human UDP-glucuronosyltransferase enzymes involved in lactonization Xenobiotica. 2003 Jan;33(1):27-41. doi: 10.1080/0049825021000017957. | ||||
| 396 | Ema.europa:Pitolisant | ||||
| 397 | Metabolism and interactions of antileprosy drugs | ||||
| 398 | Riociguat (adempas): a novel agent for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. P T. 2014 Nov;39(11):749-58. | ||||
| 399 | DrugBank(Pharmacology-Metabolism):Ruxolitinib | ||||
| 400 | Pharmacokinetics and Pharmacodynamics of Ruxolitinib: A Review | ||||
| 401 | Ruxolitinib: in the treatment of myelofibrosis. Drugs. 2012 Nov 12;72(16):2117-27. | ||||
| 402 | Effects of ketoconazole treatment on the pharmacokinetics of safinamide and its plasma metabolites in healthy adult subjects | ||||
| 403 | Disposition and metabolism of safinamide, a novel drug for Parkinson's disease, in healthy male volunteers. Pharmacology. 2013;92(3-4):207-16. | ||||
| 404 | DailyMed : Safinamide mesylate | ||||
| 405 | FDA label of Selinexor. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 406 | Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab. 2010 Aug;12(8):648-58. | ||||
| 407 | Sonidegib: first global approval. Drugs. 2015 Sep;75(13):1559-66. | ||||
| 408 | Interaction of sorafenib and cytochrome P450 isoenzymes in patients with advanced melanoma: a phase I/II pharmacokinetic interaction study. Cancer Chemother Pharmacol. 2011 Nov;68(5):1111-8. | ||||
| 409 | PubChem:Tapinarof | ||||
| 410 | The Quantification of Paclitaxel and Its Two Major Metabolites in Biological Samples by HPLC-MS/MS and Its Application in a Pharmacokinetic and Tumor Distribution Study in Xenograft Nude Mouse | ||||
| 411 | Timolol metabolism in human liver microsomes is mediated principally by CYP2D6. Drug Metab Dispos. 2007 Jul;35(7):1135-41. | ||||
| 412 | Tolterodine, a new muscarinic receptor antagonist, is metabolized by cytochromes P450 2D6 and 3A in human liver microsomes. Drug Metab Dispos. 1998 Apr;26(4):289-93. | ||||
| 413 | Effect of genetic polymorphisms in cytochrome p450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet. 2005;44(12):1209-25. | ||||
| 414 | Characterization of human cytochrome P450 isoenzymes involved in the metabolism of vinorelbine. Fundam Clin Pharmacol. 2005 Oct;19(5):545-53. | ||||
| 415 | Roles of CYP3A4 and CYP2C19 in methyl hydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem Pharmacol. 2007 Jun 15;73(12):2020-6. | ||||
| 416 | Metabolism of zaleplon by human liver: evidence for involvement of aldehyde oxidase. Xenobiotica. 2002 Oct;32(10):835-47. | ||||
| 417 | The metabolism of zidovudine by human liver microsomes in vitro: formation of 3'-amino-3'-deoxythymidine. Biochem Pharmacol. 1994 Jul 19;48(2):267-76. | ||||
| 418 | Simultaneous quantification of abemaciclib and its active metabolites in human and mouse plasma by UHPLC-MS/MS J Pharm Biomed Anal. 2021 Sep 5;203:114225. doi: 10.1016/j.jpba.2021.114225. | ||||
| 419 | Abiraterone for the treatment of metastatic castrate-resistant prostate cancer. Ann Pharmacother. 2012 Jul-Aug;46(7-8):1016-24. | ||||
| 420 | An update on the clinical pharmacology of the dipeptidyl peptidase 4 inhibitor alogliptin used for the treatment of type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015 Dec;42(12):1225-38. | ||||
| 421 | Clinical pharmacokinetics and drug-drug interactions of endothelin receptor antagonists in pulmonary arterial hypertension. J Clin Pharmacol. 2012 Dec;52(12):1784-805. | ||||
| 422 | Interaction profile of armodafinil with medications metabolized by cytochrome P450 enzymes 1A2, 3A4 and 2C19 in healthy subjects. Clin Pharmacokinet. 2008;47(1):61-74. | ||||
| 423 | DrugBank : Atenolol | ||||
| 424 | Drug Interactions with Angiotensin Receptor Blockers: Role of Human Cytochromes P450 | ||||
| 425 | Lithium Intoxication in the Elderly: A Possible Interaction between Azilsartan, Fluvoxamine, and Lithium | ||||
| 426 | Bicalutamide: clinical pharmacokinetics and metabolism. Clin Pharmacokinet. 2004;43(13):855-78. | ||||
| 427 | Pharmacokinetic evaluation of the interaction between hepatitis C virus protease inhibitor boceprevir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and pravastatin. Antimicrob Agents Chemother. 2013 Jun;57(6):2582-8. | ||||
| 428 | Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos. 2007 Aug;35(8):1400-7. | ||||
| 429 | Kinetics of dithionite-dependent reduction of cytochrome P450 3A4: heterogeneity of the enzyme caused by its oligomerization. Biochemistry. 2005 Oct 25;44(42):13902-13. | ||||
| 430 | Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy. 1998 Jan-Feb;18(1):84-112. | ||||
| 431 | Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013 Feb;9(2):193-206. | ||||
| 432 | Major role of human liver microsomal cytochrome P450 2C9 (CYP2C9) in the oxidative metabolism of celecoxib, a novel cyclooxygenase-II inhibitor. J Pharmacol Exp Ther. 2000 May;293(2):453-9. | ||||
| 433 | Effects of CYP3A inhibition on the metabolism of cilostazol. Clin Pharmacokinet. 1999;37 Suppl 2:61-8. | ||||
| 434 | In vitro metabolism of clindamycin in human liver and intestinal microsomes. Drug Metab Dispos. 2003 Jul;31(7):878-87. | ||||
| 435 | DrugBank(Pharmacology-Metabolism):Cyproterone | ||||
| 436 | Synthesis and characterization of a novel liver-targeted prodrug of cytosine-1-beta-D-arabinofuranoside monophosphate for the treatment of hepatocellular carcinoma. J Med Chem. 2006 Dec 28;49(26):7711-20. | ||||
| 437 | Metabolic interactions of putative cytochrome P4503A substrates with alternative pathways of dapsone metabolism in human liver microsomes. Drug Metab Dispos. 1996 Feb;24(2):164-171. | ||||
| 438 | Prevalence of non-cytochrome P450-mediated metabolism in food and drug administration-approved oral and intravenous drugs: 2006-2015. Drug Metab Dispos. 2016 Aug;44(8):1246-52. | ||||
| 439 | Dydrogesterone metabolism in human liver by aldo-keto reductases and cytochrome P450 enzymes. Xenobiotica. 2016 Oct;46(10):868-74. | ||||
| 440 | FDA Label of Elvitegravir. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 441 | Eplerenone: a selective aldosterone receptor antagonist for hypertension and heart failure. Heart Dis. 2003 Sep-Oct;5(5):354-63. | ||||
| 442 | Modulation of cytochrome P450 metabolism by ergonovine and dihydroergotamine. Vet Hum Toxicol. 2003 Feb;45(1):6-9. | ||||
| 443 | Activity of docetaxel with or without estramustine phosphate versus mitoxantrone in androgen dependent and independent human prostate cancer xenografts. J Urol. 2003 May;169(5):1729-34. | ||||
| 444 | Estrogen regulation of the cytochrome P450 3A subfamily in humans. J Pharmacol Exp Ther. 2004 Nov;311(2):728-35. | ||||
| 445 | News and information in drug research: Ethynodiol diacetate. | ||||
| 446 | Flibanserin: first global approval. Drugs. 2015 Oct;75(15):1815-22. | ||||
| 447 | The disposition and metabolism of [14C]fluconazole in humans Drug Metab Dispos. 1991 Jul-Aug;19(4):764-7. | ||||
| 448 | Fosamprenavir : clinical pharmacokinetics and drug interactions of the amprenavir prodrug. Clin Pharmacokinet. 2006;45(2):137-68. | ||||
| 449 | CYP1A1 is a major enzyme responsible for the metabolism of granisetron in human liver microsomes. Curr Drug Metab. 2005 Oct;6(5):469-80. | ||||
| 450 | Capillary electrophoresis contributions to the hydromorphone metabolism in man. Electrophoresis. 2006 Jun;27(12):2444-57. | ||||
| 451 | DrugBank(Pharmacology-Metabolism):Icatibant | ||||
| 452 | An updated review of iclaprim: a potent and rapidly bactericidal antibiotic for the treatment of skin and skin structure infections and nosocomial pneumonia caused by gram-positive including multidrug-resistant bacteria. Open Forum Infect Dis. 2018 Jan 6;5(2):ofy003. | ||||
| 453 | Pharmacokinetic evaluation of CYP3A4-mediated drug-drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev. 2017 Jan;6(1):44-53. | ||||
| 454 | Sensitivity of ivacaftor to drug-drug interactions with rifampin, a cytochrome P450 3A4 inducer. Pediatr Pulmonol. 2018 May;53(5):E6-E8. | ||||
| 455 | Lacosamide therapeutic monitoring in patients with epilepsy: effect of concomitant antiepileptic drugs. Ther Drug Monit. 2013 Dec;35(6):849-52. | ||||
| 456 | DrugBank(Pharmacology-Metabolism):Levomethadyl Acetate | ||||
| 457 | Differential N-demethylation of l-alpha-acetylmethadol (LAAM) and norLAAM by cytochrome P450s 2B6, 2C18, and 3A4 | ||||
| 458 | Evaluation of the effects of the weak CYP3A inhibitors atorvastatin and ethinyl estradiol/norgestimate on lomitapide pharmacokinetics in healthy subjects. J Clin Pharmacol. 2016 Jan;56(1):47-55. | ||||
| 459 | Synthesis and antiviral activities of the major metabolites of the HIV protease inhibitor ABT-378 (Lopinavir). Bioorg Med Chem Lett. 2001 Jun 4;11(11):1351-3. | ||||
| 460 | EMC: Lotemax 0.5% w/v Eye Drops, Suspension. | ||||
| 461 | Alveolar echinococcosis of the liver in an adult with human immunodeficiency virus type-1 infection. Infection. 2004 Oct;32(5):299-302. | ||||
| 462 | Effect of rifampin on plasma concentrations of mefloquine in healthy volunteers. J Pharm Pharmacol. 2000 Oct;52(10):1265-9. | ||||
| 463 | Metabolism of megestrol acetate in vitro and the role of oxidative metabolites. Xenobiotica. 2018 Oct;48(10):973-983. | ||||
| 464 | Species differences in stereoselective metabolism of mephenytoin by cytochrome P450 (CYP2C and CYP3A). J Pharmacol Exp Ther. 1993 Jan;264(1):89-94. | ||||
| 465 | Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma | ||||
| 466 | In vitro metabolism of 14C-moxidectin by hepatic microsomes from various species. Vet Res Commun. 2001 Jul;25(5):345-54. | ||||
| 467 | Inhibitory effects of nicardipine to cytochrome P450 (CYP) in human liver microsomes. Biol Pharm Bull. 2005 May;28(5):882-5. | ||||
| 468 | Metabolic interactions of selected antimalarial and non-antimalarial drugs with the major pathway (3-hydroxylation) of quinine in human liver microsomes. Br J Clin Pharmacol. 1997 Nov;44(5):505-11. | ||||
| 469 | Orphenadrine and methimazole inhibit multiple cytochrome P450 enzymes in human liver microsomes. Drug Metab Dispos. 1997 Mar;25(3):390-3. | ||||
| 470 | Bioreactor systems in drug metabolism: synthesis of cytochrome P450-generated metabolites. Metab Eng. 2000 Apr;2(2):115-25. | ||||
| 471 | Safety, tolerability and pharmacokinetic profile of single and multiple oral doses of arterolane (RBx11160) maleate in healthy subjects | ||||
| 472 | ABCB1 polymorphisms influence steady-state plasma levels of 9-hydroxyrisperidone and risperidone active moiety. Ther Drug Monit. 2008 Oct;30(5):628-33. | ||||
| 473 | FDA Label of Qsymia. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 474 | The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs. 2012 Nov 12;72(16):2087-116. | ||||
| 475 | FDA briefing document - pretomanid tablet, 200 mg meeting of the antimicrobial drugs advisory committee (AMDAC). | ||||
| 476 | The metabolism of pyrazoloacridine (NSC 366140) by cytochromes p450 and flavin monooxygenase in human liver microsomes. Clin Cancer Res. 2004 Feb 15;10(4):1471-80. | ||||
| 477 | Interaction between grapefruit juice and hypnotic drugs: comparison of triazolam and quazepam. Eur J Clin Pharmacol. 2006 Mar;62(3):209-15. | ||||
| 478 | Effects of acid and lactone forms of eight HMG-CoA reductase inhibitors on CYP-mediated metabolism and MDR1-mediated transport. Pharm Res. 2006 Mar;23(3):506-12. | ||||
| 479 | Clinical pharmacokinetics of salmeterol. Clin Pharmacokinet. 2002;41(1):19-30. | ||||
| 480 | In vitro metabolic profile and drug-drug interaction assessment of secnidazole, a high-dose 5-nitroimidazole antibiotic for the treatment of bacterial vaginosis Pharmacol Res Perspect. 2020 Aug;8(4):e00634. doi: 10.1002/prp2.634. | ||||
| 481 | Physiologically Based Pharmacokinetic Modeling of Transdermal Selegiline and Its Metabolites for the Evaluation of Disposition Differences between Healthy and Special Populations | ||||
| 482 | P450 phenotyping of the metabolism of selegiline to desmethylselegiline and methamphetamine. Drug Metab Pharmacokinet. 2007 Apr;22(2):78-87. | ||||
| 483 | FDA LABEL:elexipag | ||||
| 484 | Sibutramine: a serotonin-norepinephrine reuptake-inhibitor for the treatment of obesity. Ann Pharmacother. 1999 Sep;33(9):968-78. | ||||
| 485 | Product characteristics of Diacomit. | ||||
| 486 | Studies on the metabolism and biological activity of the epimers of sulindac | ||||
| 487 | Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study | ||||
| 488 | Sigmoidal kinetic model for two co-operative substrate-binding sites in a cytochrome P450 3A4 active site: an example of the metabolism of diazepam and its derivatives. Biochem J. 1999 Jun 15;340 ( Pt 3):845-53. | ||||
| 489 | The In vitro hepatic metabolism of quinine in mice, rats and dogs: comparison with human liver microsomes. J Pharmacol Exp Ther. 1997 Dec;283(3):1168-76. | ||||
| 490 | Enzyme induction and inhibition by new antiepileptic drugs: a review of human studies. Fundam Clin Pharmacol. 2000 Jul-Aug;14(4):301-19. | ||||
| 491 | FDA label of Tinidazole. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 492 | Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD. Treat Respir Med. 2004;3(4):247-68. | ||||
| 493 | Tolvaptan: a new therapeutic agent. Rev Recent Clin Trials. 2011 May;6(2):177-88. | ||||
| 494 | In vitro drug-drug interaction potential of sulfoxide and/or sulfone metabolites of albendazole, triclabendazole, aldicarb, methiocarb, montelukast and ziprasidone. Drug Metab Lett. 2018;12(2):101-116. | ||||
| 495 | The clinical pharmacology and pharmacokinetics of ulipristal acetate for the treatment of uterine fibroids. Reprod Sci. 2015 Apr;22(4):476-83. | ||||
| 496 | FDA label of Vandetanib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 497 | Vorapaxar: the missing link in antiplatelet therapy! J Anaesthesiol Clin Pharmacol. 2017 Apr-Jun;33(2):269-270. | ||||
| 498 | Pharmacokinetics and drug interactions with zonisamide. Epilepsia. 2007 Mar;48(3):435-41. | ||||
| 499 | LABEL:Avacopan | ||||
| 500 | Aztreonam decreases hepatic microsomal cytochrome P450 in cynomolgus monkeys. Pharmacology. 1994 Mar;48(3):137-42. | ||||
| 501 | FDA Label of Baloxavir marboxil. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 502 | Pharmacokinetics and safety of a novel influenza treatment (baloxavir marboxil) in Korean subjects compared with Japanese subjects | ||||
| 503 | Product monograph: LUMIGAN RC (Bimatoprost). | ||||
| 504 | Stereoselective metabolism of bisoprolol enantiomers in dogs and humans. Life Sci. 1998;63(13):1097-108. | ||||
| 505 | Cytochrome P450 2C8 and CYP3A4/5 are involved in chloroquine metabolism in human liver microsomes. Arch Pharm Res. 2003 Aug;26(8):631-7. | ||||
| 506 | Metabolism and Interactions of Chloroquine and Hydroxychloroquine with Human Cytochrome P450 Enzymes and Drug Transporters Curr Drug Metab. 2020;21(14):1127-1135. doi: 10.2174/1389200221999201208211537. | ||||
| 507 | Integrated analysis on the physicochemical properties of dihydropyridine calcium channel blockers in grapefruit juice interactions. Curr Pharm Biotechnol. 2012 Jul;13(9):1705-17. | ||||
| 508 | Tacrolimus interaction with clotrimazole: a concise case report and literature review. P T. 2010 Oct;35(10):568-9. | ||||
| 509 | Conivaptan: a dual vasopressin receptor v1a/v2 antagonist [corrected]. Cardiovasc Drug Rev. 2007 Fall;25(3):261-79. | ||||
| 510 | Phase 1 study to investigate the pharmacokinetic properties of dacomitinib in healthy adult Chinese subjects genotyped for CYP2D6. Xenobiotica. 2018 May;48(5):459-466. | ||||
| 511 | Risk of stroke, gangrene from ergot drug interactions | ||||
| 512 | Drug-Drug interactions of clinical significance in the treatment of patients with Mycobacterium avium complex disease. Clin Pharmacokinet. 2000 Sep;39(3):203-14. | ||||
| 513 | Species difference in stereoselective involvement of CYP3A in the mono-N-dealkylation of disopyramide. Xenobiotica. 2001 Feb;31(2):73-83. | ||||
| 514 | Clinical pharmacokinetics and drug-drug interactions of Elbasvir/Grazoprevir. Eur J Drug Metab Pharmacokinet. 2018 Oct;43(5):509-531. | ||||
| 515 | FDA:Enasidenib | ||||
| 516 | Development of encorafenib for BRAF-mutated advanced melanoma. Curr Opin Oncol. 2018 Mar;30(2):125-133. | ||||
| 517 | Metabolism of epinastine, a histamine H1 receptor antagonist, in human liver microsomes in comparison with that of terfenadine. Res Commun Mol Pathol Pharmacol. 1997 Dec;98(3):273-92. | ||||
| 518 | Effect of the CYP3A4 inhibitor erythromycin on the pharmacokinetics of lignocaine and its pharmacologically active metabolites in subjects with normal and impaired liver function. Br J Clin Pharmacol. 2003 Jan;55(1):86-93. | ||||
| 519 | Alkylation of the prosthetic heme in cytochrome P-450 during oxidative metabolism of the sedative-hypnotic ethchlorvynol. J Med Chem. 1982 Oct;25(10):1174-9. | ||||
| 520 | Drug interactions with calcium channel blockers: possible involvement of metabolite-intermediate complexation with CYP3A. Drug Metab Dispos. 2000 Feb;28(2):125-30. | ||||
| 521 | NCIthesaurus: Fluprednisolone | ||||
| 522 | The inhaled glucocorticoid fluticasone propionate efficiently inactivates cytochrome P450 3A5, a predominant lung P450 enzyme. Chem Res Toxicol. 2010 Aug 16;23(8):1356-64. | ||||
| 523 | Fluticasone propionate pharmacogenetics: CYP3A4*22 polymorphism and pediatric asthma control | ||||
| 524 | DrugBank(Pharmacology-Metabolism):Fosinopril | ||||
| 525 | Fulvestrant: pharmacologic profile versus existing endocrine agents for the treatment of breast cancer. Ann Pharmacother. 2006 Sep;40(9):1572-83. | ||||
| 526 | Evaluation of Rifampin's Transporter Inhibitory and CYP3A Inductive Effects on the Pharmacokinetics of Venetoclax, a BCL-2 Inhibitor: Results of a Single- and Multiple-Dose Study | ||||
| 527 | Tenofovir alafenamide in the treatment of chronic hepatitis B virus infection: rationale and clinical trial evidence | ||||
| 528 | NCIthesaurus: Halcinonide | ||||
| 529 | Pharmacokinetic and pharmacodynamic interaction between grapefruit juice and halofantrine. Clin Pharmacol Ther. 2002 Nov;72(5):514-23. | ||||
| 530 | Stereoselective method development and validation for determination of concentrations of amphetamine-type stimulants and metabolites in human urine using a simultaneous extraction-chiral derivatization approach. J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Jan 1;879(1):8-16. | ||||
| 531 | Hydroxychloroquine: a physiologically-based pharmacokinetic model in the context of cancer-related autophagy modulation. J Pharmacol Exp Ther. 2018 Jun;365(3):447-459. | ||||
| 532 | Prevention of preterm delivery with 17-hydroxyprogesterone caproate: pharmacologic considerations. Semin Perinatol. 2014 Dec;38(8):516-22. | ||||
| 533 | Product characteristics of HYDROXYZINE (Hydroxyzine Hydrochloride Capsules). | ||||
| 534 | Cross-species comparisons of the pharmacokinetics of ibudilast | ||||
| 535 | Use of indacaterol for the treatment of COPD: a pharmacokinetic evaluation. Expert Opin Drug Metab Toxicol. 2014 Jan;10(1):129-37. | ||||
| 536 | Isavuconazole: pharmacology, pharmacodynamics, and current clinical experience with a new triazole antifungal agent. Pharmacotherapy. 2015 Nov;35(11):1037-51. | ||||
| 537 | Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy. 2018 Sep;38(9):935-946. | ||||
| 538 | FDA label:Lenacapavir | ||||
| 539 | DrugBank(Pharmacology-Metabolism):Lenacapavir | ||||
| 540 | Inhibition of drug metabolizing cytochrome P450s by the aromatase inhibitor drug letrozole and its major oxidative metabolite 4,4'-methanol-bisbenzonitrile in vitro. Cancer Chemother Pharmacol. 2009 Oct;64(5):867-75. | ||||
| 541 | Identification of human liver cytochrome P450 enzymes responsible for the metabolism of lonafarnib (Sarasar). Drug Metab Dispos. 2006 Apr;34(4):628-35. | ||||
| 542 | Identification of unstable metabolites of Lonafarnib using liquid chromatography-quadrupole time-of-flight mass spectrometry, stable isotope incorporation and ion source temperature alteration | ||||
| 543 | FDA label of Lorlatinib. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 544 | Development of a paediatric physiologically based pharmacokinetic model to assess the impact of drug-drug interactions in tuberculosis co-infected malaria subjects: A case study with artemether-lumefantrine and the CYP3A4-inducer rifampicin. Eur J Pharm Sci. 2017 Aug 30;106:20-33. | ||||
| 545 | Activation of human cytochrome P-450 3A4-catalyzed meloxicam 5'-methylhydroxylation by quinidine and hydroquinidine in vitro. J Pharmacol Exp Ther. 1999 Jul;290(1):1-8. | ||||
| 546 | DrugBank(Pharmacology-Metabolism):Methysergide | ||||
| 547 | DrugBank(Pharmacology-Metabolism):Mitapivat | ||||
| 548 | Evaluation of 24 CYP2D6 variants on the metabolism of nebivolol in vitro. Drug Metab Dispos. 2016 Nov;44(11):1828-1831. | ||||
| 549 | Metabolite identification, reaction phenotyping, and retrospective drug-drug interaction predictions of 17-deacetylnorgestimate, the active component of the oral contraceptive norgestimate. Drug Metab Dispos. 2017 Jun;45(6):676-685. | ||||
| 550 | Cytochrome P-450 activities in human and rat brain microsomes. Brain Res. 2000 Feb 14;855(2):235-43. | ||||
| 551 | PubChem:Omidenepag isopropyl | ||||
| 552 | DrugBank(Pharmacology-Metabolism):Orlistat | ||||
| 553 | A comparative review of oxybutynin chloride formulations: pharmacokinetics and therapeutic efficacy in overactive bladder. Rev Urol. 2010 Winter;12(1):12-9. | ||||
| 554 | The Effect of Respiratory Protective Surgical Mask on Physiological Markers of Endurance Performance in a Recreational Runner | ||||
| 555 | Evaluation of drug-drug interactions of pemigatinib in healthy participants | ||||
| 556 | Evaluation of the pharmacokinetics of pemigatinib in patients with impaired hepatic or renal function | ||||
| 557 | Evaluation of drug-drug interaction potential for pemigatinib using physiologically based pharmacokinetic modeling | ||||
| 558 | Human metabolic interactions of environmental chemicals. J Biochem Mol Toxicol. 2007;21(4):182-6. | ||||
| 559 | Pimecrolimus: absorption, distribution, metabolism, and excretion in healthy volunteers after a single oral dose and supplementary investigations in vitro. Drug Metab Dispos. 2006 May;34(5):765-74. | ||||
| 560 | Survey of computed tomography doses and establishment of national diagnostic reference levels in the R | ||||
| 561 | Biotransformation of praziquantel by human cytochrome p450 3A4 (CYP 3A4). Acta Pol Pharm. 2006 Sep-Oct;63(5):381-5. | ||||
| 562 | HEP Drug interaction: daclatasvir and abacavir. | ||||
| 563 | Ketoconazole and rifampin significantly affect the pharmacokinetics, but not the safety or QTc interval, of casopitant, a neurokinin-1 receptor antagonist. J Clin Pharmacol. 2010 Aug;50(8):951-9. | ||||
| 564 | Inhibitory potential of twenty five anti-tuberculosis drugs on CYP activities in human liver microsomes. Biol Pharm Bull. 2015;38(9):1425-9. | ||||
| 565 | U. S. FDA Label -Rifaximin | ||||
| 566 | PubChem:Rimegepant | ||||
| 567 | Effects of rimegepant 75?mg daily on the pharmacokinetics of a combined oral contraceptive containing ethinyl estradiol and norgestimate in healthy female participants | ||||
| 568 | Identification of clinically used drugs that activate pregnane X receptors. Drug Metab Dispos. 2011 Jan;39(1):151-9. | ||||
| 569 | Effects of CYP3A Inhibition, CYP3A Induction, and Gastric Acid Reduction on the Pharmacokinetics of Ripretinib, a Switch Control KIT Tyrosine Kinase Inhibitor | ||||
| 570 | Estimation of FMO3 Ontogeny by Mechanistic Population Pharmacokinetic Modelling of Risdiplam and Its Impact on Drug-Drug Interactions in Children | ||||
| 571 | Effect of steady-state enoxacin on single-dose pharmacokinetics of roflumilast and roflumilast N-oxide. J Clin Pharmacol. 2011 Apr;51(4):586-93. | ||||
| 572 | Pharmacokinetic interactions of rolapitant with cytochrome P450 3A substrates in healthy subjects. J Clin Pharmacol. 2019 Apr;59(4):488-499. | ||||
| 573 | A phase I/II trial of the histone deacetylase inhibitor romidepsin for adults with recurrent malignant glioma: North American Brain Tumor Consortium Study 03-03. Neuro Oncol. 2011 May;13(5):509-16. | ||||
| 574 | Effect of rifampicin on the pharmacokinetics and pharmacodynamics of saxagliptin, a dipeptidyl peptidase-4 inhibitor, in healthy subjects. Br J Clin Pharmacol. 2011 Jul;72(1):92-102. | ||||
| 575 | P-Glycoprotein (ABCB1/MDR1) and BCRP (ABCG2) Limit Brain Accumulation and Cytochrome P450-3A (CYP3A) Restricts Oral Exposure of the RET Inhibitor Selpercatinib (RETEVMO) | ||||
| 576 | Overview of Drug-Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Targeted Therapy and Supportive Care for Lung Cancer | ||||
| 577 | Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology. 1993 Oct;79(4):795-807. | ||||
| 578 | Hepatitis C virus direct-acting antiviral drug interactions and use in renal and hepatic impairment. Top Antivir Med. 2015 May-Jun;23(2):92-6. | ||||
| 579 | Possible involvement of multiple cytochrome P450S in fentanyl and sufentanil metabolism as opposed to alfentanil. Biochem Pharmacol. 1997 Jun 1;53(11):1613-9. | ||||
| 580 | Contribution of CYP3A isoforms to dealkylation of PDE5 inhibitors: a comparison between sildenafil N-demethylation and tadalafil demethylenation. Biol Pharm Bull. 2015;38(1):58-65. | ||||
| 581 | FDA label of Tenapanor. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 582 | FDA label of Bictegravir, emtricitabine, and tenofovir alafenamide. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 583 | Digging deeper into CYP3A testosterone metabolism: kinetic, regioselectivity, and stereoselectivity differences between CYP3A4/5 and CYP3A7. Drug Metab Dispos. 2017 Dec;45(12):1266-1275. | ||||
| 584 | In vitro metabolism of the opioid tilidine and interaction of tilidine and nortilidine with CYP3A4, CYP2C19, and CYP2D6 | ||||
| 585 | Triamcinolone acetonide induced secondary adrenal insufficiency related to impaired CYP3A4 metabolism by coadministration of nefazodone. Pain Med. 2010 Jul;11(7):1132-5. | ||||
| 586 | Long-term low-dose ketoconazole treatment in bilateral macronodular adrenal hyperplasia. Endocrinol Diabetes Metab Case Rep. 2014;2014:140083. | ||||
| 587 | Involvement of cytochrome P450 2C9, 2E1 and 3A4 in trimethadione N-demethylation in human microsomes. J Clin Pharm Ther. 2003 Dec;28(6):493-6. | ||||
| 588 | Effects of roxithromycin, erythromycin and troleandomycin on their N-demethylation by rat and human cytochrome P450 enzymes. Xenobiotica. 1996 Nov;26(11):1143-53. | ||||
| 589 | Erectile dysfunction drugs changed the protein expressions and activities of drug-metabolising enzymes in the liver of male rats. Oxid Med Cell Longev. 2016;2016:4970906. | ||||
| 590 | Product characteristics of Epclusa. | ||||
| 591 | Preclinical Pharmacokinetics and First-in-Human Pharmacokinetics, Safety, and Tolerability of Velpatasvir, a Pangenotypic Hepatitis C Virus NS5A Inhibitor, in Healthy Subjects | ||||
| 592 | Induction of CYP3A4 by vinblastine: role of the nuclear receptor NR1I2. Ann Pharmacother. 2010 Nov;44(11):1709-17. | ||||
| 593 | Association of CYP3A5 expression and vincristine neurotoxicity in pediatric malignancies in Turkish population. J Pediatr Hematol Oncol. 2017 Aug;39(6):458-462. | ||||
| 594 | Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J Steroid Biochem Mol Biol. 2013 Jul;136:54-8. | ||||
| 595 | FDA Label of Vosevi. The 2020 official website of the U.S. Food and Drug Administration | ||||
| 596 | DrugBank(Pharmacology-Metabolism):Voxilaprevir | ||||
| 597 | Protease inhibitors in patients with HIV disease. Clinically important pharmacokinetic considerations. Clin Pharmacokinet. 1997 Mar;32(3):194-209. | ||||
| 598 | Prediction of cytochrome P450 isoform responsible for metabolizing a drug molecule. BMC Pharmacol. 2010 Jul 16;10:8. | ||||
| 599 | DrugBank(Pharmacology-Metabolism):Alfuzosin | ||||
| 600 | U. S. FDA Label -Alfuzosin | ||||
| 601 | Time-dependent pharmacokinetics and drug metabolism of atovaquone plus proguanil (Malarone) when taken as chemoprophylaxis. Eur J Clin Pharmacol. 2002 Apr;58(1):19-27. | ||||
| 602 | Transport and metabolic characterization of Caco-2 cells expressing CYP3A4 and CYP3A4 plus oxidoreductase. Pharm Res. 1999 Sep;16(9):1352-9. | ||||
| 603 | Cytochrome p450: a target for drug development for skin diseases. J Invest Dermatol. 2004 Sep;123(3):417-25. | ||||
| 604 | Troglitazone induces CYP3A4 activity leading to falsely abnormal dexamethasone suppression test. J Clin Endocrinol Metab. 2003 Jul;88(7):3113-6. | ||||
| 605 | Progress in Brain Research. Chapter 11 - Steroidogenic Enzymes in the Brain: Morphological Aspects. 2010, 181:193-207. | ||||
| 606 | A further interaction study of quinine with clinically important drugs by human liver microsomes: determinations of inhibition constant (Ki) and type of inhibition. Eur J Drug Metab Pharmacokinet. 1999 Jul-Sep;24(3):272-8. | ||||
| 607 | DrugBank(Pharmacology-Metabolism):Ergoloid mesylate | ||||
| 608 | Delineation of the interactions between the chemotherapeutic agent eribulin mesylate (E7389) and human CYP3A4. Cancer Chemother Pharmacol. 2008 Sep;62(4):707-16. | ||||
| 609 | Eribulin, a microtubule inhibitor for metastatic breast cancer. Oncology (Williston Park). 2011 Feb;25(2 Suppl Nurse Ed):46-8. | ||||
| 610 | The pharmacological interactions between direct-acting antivirals for the treatment of chronic hepatitis c and psychotropic drugs. Expert Rev Clin Pharmacol. 2018 Oct;11(10):999-1030. | ||||
| 611 | DrugBank(Pharmacology-Metabolism):IPI-145 | ||||
| 612 | A basic conceptual and practical overview of interactions with highly prescribed drugs. Can J Clin Pharmacol. 2002 Winter;9(4):191-8. | ||||
| 613 | [Macrolides and ketolides] | ||||
| 614 | Photochemotherapeutic agent 8-methoxypsoralen induces cytochrome P450 3A4 and carboxylesterase HCE2: evidence on an involvement of the pregnane X receptor. Toxicol Sci. 2007 Jan;95(1):13-22. | ||||
| 615 | Inhibition of cytochrome P450 enzymes participating in p-nitrophenol hydroxylation by drugs known as CYP2E1 inhibitors. Chem Biol Interact. 2004 Apr 15;147(3):331-40. | ||||
| 616 | Delta-9-Tetrahydrocannabinol and Cannabidiol Drug-Drug Interactions | ||||
| 617 | DrugBank(Pharmacology-Metabolism):NERATINIB MALEATE | ||||
| 618 | Characterization of in vitro metabolites of deoxypodophyllotoxin in human and rat liver microsomes using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(1):52-8. | ||||
| 619 | Metabolism of ramelteon in human liver microsomes and correlation with the effect of fluvoxamine on ramelteon pharmacokinetics. Drug Metab Dispos. 2010 Aug;38(8):1381-91. | ||||
| 620 | Structural characterization of the homotropic cooperative binding of azamulin to human cytochrome P450 3A5 | ||||
| 621 | P-glycoprotein limits ribociclib brain exposure and CYP3A4 restricts its oral bioavailability. Mol Pharm. 2019 Sep 3;16(9):3842-3852. | ||||
| 622 | Characterization of testosterone 11 beta-hydroxylation catalyzed by human liver microsomal cytochromes P450 | ||||
| 623 | FDA label of Trifarotene. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 624 | Pharmacokinetic Drug-Drug Interaction Studies Between Trilaciclib and Midazolam, Metformin, Rifampin, Itraconazole, and Topotecan in Healthy Volunteers and Patients with Extensive-Stage Small-Cell Lung Cancer | ||||
| 625 | Umbralisib: First Approval | ||||
| 626 | Population pharmacokinetics of upadacitinib using the immediate-release and extended-release formulations in healthy subjects and subjects with rheumatoid arthritis: analyses of phase I-III clinical trials. Clin Pharmacokinet. 2019 Aug;58(8):1045-1058. | ||||
| 627 | PubChem:Upadacitinib | ||||
| 628 | PubChem:Voclosporin | ||||
| 629 | Lestaurtinib, a multitargeted tyrosine kinase inhibitor: from bench to bedside | ||||
| 630 | In vitro identification of metabolic pathways and cytochrome P450 enzymes involved in the metabolism of etoperidone. Xenobiotica. 2002 Nov;32(11):949-62. | ||||
| 631 | CYP2D plays a major role in berberine metabolism in liver of mice and humans | ||||
| 632 | The involvement of CYP3A4 and CYP2C9 in the metabolism of 17 alpha-ethinylestradiol. Drug Metab Dispos. 2004 Nov;32(11):1209-12. | ||||
| 633 | The involvement of flavin-containing monooxygenase but not CYP3A4 in metabolism of itopride hydrochloride, a gastroprokinetic agent: comparison with cisapride and mosapride citrate. Drug Metab Dispos. 2000 Oct;28(10):1231-7. | ||||
| 634 | Effects of Dexmedetomidine on the Pharmacokinetics of Parecoxib and Its Metabolite Valdecoxib in Beagles by UPLC-MS/MS. Biomed Res Int. 2020 Aug 6;2020:1563874. doi: 10.1155/2020/1563874. | ||||
| 635 | Clinical pharmacokinetics of reboxetine, a selective norepinephrine reuptake inhibitor for the treatment of patients with depression. Clin Pharmacokinet. 2000 Dec;39(6):413-27. | ||||
| 636 | Characterization of ebastine, hydroxyebastine, and carebastine metabolism by human liver microsomes and expressed cytochrome P450 enzymes: major roles for CYP2J2 and CYP3A. Drug Metab Dispos. 2006 Nov;34(11):1793-7. | ||||
| 637 | Metabolic drug interactions with newer antipsychotics: a comparative review. Basic Clin Pharmacol Toxicol. 2007 Jan;100(1):4-22. | ||||
| 638 | Inhibition of the metabolism of brotizolam by erythromycin in humans: in vivo evidence for the involvement of CYP3A4 in brotizolam metabolism. Br J Clin Pharmacol. 2005 Aug;60(2):172-5. | ||||
| 639 | Progestogens in menopausal hormone therapy. Prz Menopauzalny. 2015 Jun;14(2):134-43. | ||||
| 640 | Prescribrt's digital referenve - Ethinyl estradiol/norethindrone; mestranol/norethindrone - Drug Summary | ||||
| 641 | Antimalarial artemisinin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol. 2005 Jun;67(6):1954-65. | ||||
| 642 | Pharmacokinetics of single and multiple escalating doses of dapoxetine in healthy volunteers. Clinical Pharmacology Therapeutics, 2004, 75(2):P32. | ||||
| 643 | Examination of metabolic pathways and identification of human liver cytochrome P450 isozymes responsible for the metabolism of barnidipine, a calcium channel blocker. Xenobiotica. 1997 Sep;27(9):885-900. | ||||
| 644 | Characterisation of seven medications approved for attention-deficit/hyperactivity disorder using in?vitro models of hepatic metabolism | ||||
| 645 | Ketobemidone is a substrate for cytochrome P4502C9 and 3A4, but not for P-glycoprotein. Xenobiotica. 2005 Aug;35(8):785-96. | ||||
| 646 | Metabolism of MK-0524, a prostaglandin D2 receptor 1 antagonist, in microsomes and hepatocytes from preclinical species and humans | ||||
| 647 | Effect of mibefradil on CYP3A4 in vivo. J Clin Pharmacol. 2003 Oct;43(10):1091-100. | ||||
| 648 | No influence of the CYP2C19-selective inhibitor omeprazole on the pharmacokinetics of the dopamine receptor agonist rotigotine | ||||
| 649 | Characterization of human urinary metabolites of the antimalarial piperaquine | ||||
| 650 | Metabolite identification of the antimalarial piperaquine in vivo using liquid chromatography-high-resolution mass spectrometry in combination with multiple data-mining tools in tandem | ||||
| 651 | In vitro metabolism of piperaquine is primarily mediated by CYP3A4 | ||||
| 652 | Stereoselective metabolism of cibenzoline, an antiarrhythmic drug, by human and rat liver microsomes: possible involvement of CYP2D and CYP3A. Drug Metab Dispos. 2000 Sep;28(9):1128-34. | ||||
| 653 | Assessment of the stereoselective metabolism of methaqualone in man by capillary electrophoresis. Electrophoresis. 2003 Aug;24(15):2598-607. | ||||
| 654 | Product monograph: Mictoryl (Propiverine hydrochloride modified-release capsules). | ||||
| 655 | Hepatic veno-occlusive disease associated with immunosuppressive cyclophosphamide dosing and roxithromycin. Ann Pharmacother. 2006 Apr;40(4):767-70. | ||||
| 656 | In vitro metabolism of TAK-438, vonoprazan fumarate, a novel potassium-competitive acid blocker. Xenobiotica. 2017 Dec;47(12):1027-1034. | ||||
| 657 | Assessments of CYP?inhibition?based drug-drug interaction between vonoprazan and poziotinib in?vitro and in?vivo | ||||
| 658 | New antipsychotic agents for schizophrenia: pharmacokinetics and metabolism update. Curr Opin Investig Drugs. 2002 Jul;3(7):1073-80. | ||||
| 659 | CYP3A is responsible for N-dealkylation of haloperidol and bromperidol and oxidation of their reduced forms by human liver microsomes. Life Sci. 2000 Nov 3;67(24):2913-20. | ||||
| 660 | Pharmacokinetics of dihydrocodeine and its active metabolite after single and multiple oral dosing. Br J Clin Pharmacol. 1999 Sep;48(3):317-22. | ||||
| 661 | Role of human liver cytochrome P4503A in the metabolism of etoricoxib, a novel cyclooxygenase-2 selective inhibitor. Drug Metab Dispos. 2001 Jun;29(6):813-20. | ||||
| 662 | Differences in the In Vivo and In Vitro Metabolism of Imrecoxib in Humans: Formation of the Rate-Limiting Aldehyde Intermediate Drug Metab Dispos. 2018 Sep;46(9):1320-1328. doi: 10.1124/dmd.118.081182. | ||||
| 663 | Metabolism of ipecac alkaloids cephaeline and emetine by human hepatic microsomal cytochrome P450s, and their inhibitory effects on P450 enzyme activities. Biol Pharm Bull. 2001 Jun;24(6):678-82. | ||||
| 664 | The effects of lacidipine on the steady/state plasma concentrations of simvastatin in healthy subjects. Br J Clin Pharmacol. 2001 Feb;51(2):147-52. | ||||
| 665 | Involvement of CYP2E1 as A low-affinity enzyme in phenacetin O-deethylation in human liver microsomes. Drug Metab Dispos. 1999 Aug;27(8):860-5. | ||||
| 666 | Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet. 2005;44(3):279-304. | ||||
| 667 | Tianeptine: a facilitator of the reuptake of serotonin and norepinephrine as an antidepressant? J Psychiatr Pract. 2004 Sep;10(5):323-30. | ||||
| 668 | Contribution of human hepatic cytochrome P450s and steroidogenic CYP17 to the N-demethylation of aminopyrine. Xenobiotica. 1999 Feb;29(2):187-93. | ||||
| 669 | Contribution of human hepatic cytochrome p450 isoforms to the metabolism of psychotropic drugs. Biol Pharm Bull. 2005 Sep;28(9):1711-6. | ||||
| 670 | CYP3A4 mediates dextropropoxyphene N-demethylation to nordextropropoxyphene: human in vitro and in vivo studies and lack of CYP2D6 involvement. Xenobiotica. 2004 Oct;34(10):875-87. | ||||
| 671 | Cytochrome P450 3A4 and benzodiazepines. Seishin Shinkeigaku Zasshi. 2003;105(5):631-42. | ||||
| 672 | Effect of an oral contraceptive preparation containing ethinylestradiol and gestodene on CYP3A4 activity as measured by midazolam 1'-hydroxylation. Br J Clin Pharmacol. 2000 Oct;50(4):333-7. | ||||
| 673 | Adverse drug interactions: a handbook for prescribers. Taylor and Francis group.2010. | ||||
| 674 | The metabolism of the piperazine-type phenothiazine neuroleptic perazine by the human cytochrome P-450 isoenzymes. Eur Neuropsychopharmacol. 2004 May;14(3):199-208. | ||||
| 675 | Eszopiclone, a nonbenzodiazepine sedative-hypnotic agent for the treatment of transient and chronic insomnia. Clin Ther. 2006 Apr;28(4):491-516. | ||||
| 676 | KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (DRUG:D01145) | ||||
| 677 | Characterization of benidipine and its enantiomers' metabolism by human liver cytochrome P450 enzymes. Drug Metab Dispos. 2007 Sep;35(9):1518-24. | ||||
| 678 | CYP2D6 is primarily responsible for the metabolism of clomiphene. Drug Metab Pharmacokinet. 2008;23(2):101-5. | ||||
| 679 | Inhibition of the metabolism of etizolam by itraconazole in humans: evidence for the involvement of CYP3A4 in etizolam metabolism. Eur J Clin Pharmacol. 2004 Aug;60(6):427-30. | ||||
| 680 | Characterization of kinetics of human cytochrome P450s involved in bioactivation of flucloxacillin: inhibition of CYP3A-catalysed hydroxylation by sulfaphenazole. Br J Pharmacol. 2019 Feb;176(3):466-477. | ||||
| 681 | FDA label of Elbasvir and grazoprevir. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 682 | Metabolism of (S)-5,6-difluoro-4-cyclopropylethynyl-4-trifluoromethyl-3, 4-dihydro-2(1H)-quinazolinone, a non-nucleoside reverse transcriptase inhibitor, in human liver microsomes. Metabolic activation and enzyme kinetics. Drug Metab Dispos. 2003 Jan;31(1):122-32. | ||||
| 683 | Pharmacologic Characterization of Valbenazine (NBI-98854) and Its Metabolites J Pharmacol Exp Ther. 2017 Jun;361(3):454-461. doi: 10.1124/jpet.116.239160. | ||||
| 684 | Excretion and metabolism of milnacipran in humans after oral administration of milnacipran hydrochloride Drug Metab Dispos. 2012 Sep;40(9):1723-35. doi: 10.1124/dmd.112.045120. | ||||
| 685 | Effect of clarithromycin on the pharmacokinetics of pranlukast in healthy volunteers. Drug Metab Pharmacokinet. 2008;23(6):428-33.; | ||||
| 686 | Cytochrome P450 expression and regulation in CYP3A4/CYP2D6 double transgenic humanized mice. Drug Metab Dispos. 2008 Feb;36(2):435-41. | ||||
| 687 | In vitro proguanil activation to cycloguanil is mediated by CYP2C19 and CYP3A4 in adult Chinese liver microsomes. Acta Pharmacol Sin. 2000 Aug;21(8):747-52. | ||||
| 688 | Rupatadine: pharmacological profile and its use in the treatment of allergic disorders. Expert Opin Pharmacother. 2006 Oct;7(14):1989-2001. | ||||
| 689 | Application of static models to predict midazolam clinical interactions in the presence of single or multiple hepatitis C virus drugs. Drug Metab Dispos. 2016 Aug;44(8):1372-80. | ||||
| 690 | Rational prescription of drugs within similar therapeutic or structural class for gastrointestinal disease treatment: drug metabolism and its related interactions. World J Gastroenterol. 2007 Nov 14;13(42):5618-28. | ||||
| 691 | Protein binding and the metabolism of thiamylal enantiomers in vitro. Anesth Analg. 2000 Sep;91(3):736-40. | ||||
| 692 | Drug interactions between rifamycin antibiotics and hormonal contraception: a systematic review. BJOG. 2018 Jun;125(7):804-811. | ||||
| 693 | Human sympathetic activation by alpha2-adrenergic blockade with yohimbine: bimodal, epistatic influence of cytochrome P450-mediated drug metabolism. Clin Pharmacol Ther. 2004 Aug;76(2):139-53. | ||||
| 694 | Evidence for the bioactivation of zomepirac and tolmetin by an oxidative pathway: identification of glutathione adducts in vitro in human liver microsomes and in vivo in rats. Drug Metab Dispos. 2006 Jan;34(1):145-51. | ||||
| 695 | Isoform-specific regulation of cytochromes P450 expression by estradiol and progesterone. Drug Metab Dispos. 2013 Feb;41(2):263-9. | ||||
| 696 | Preclinical pharmacokinetics and disposition of a novel selective VEGFR inhibitor fruquintinib (HMPL-013) and the prediction of its human pharmacokinetics | ||||
| 697 | Physiologically Based Pharmacokinetic Modeling for Selumetinib to Evaluate Drug-Drug Interactions and Pediatric Dose Regimens | ||||
| 698 | Metabolism and excretion of anacetrapib, a novel inhibitor of the cholesteryl ester transfer protein, in humans. Drug Metab Dispos. 2010 Mar;38(3):474-83. | ||||
| 699 | Human liver S9 fractions: metabolism of dehydroepiandrosterone, epiandrosterone, and related 7-hydroxylated derivatives | ||||
| 700 | Pharmacokinetics of asciminib in the presence of CYP3A or P-gp inhibitors, CYP3A inducers, and acid-reducing agents | ||||
| 701 | Absorption, metabolism and excretion of [14C]gemigliptin, a novel dipeptidyl peptidase 4 inhibitor, in humans | ||||
| 702 | Stable expression of human cytochrome P450 3A4 in V79 cells and its application for metabolic profiling of ergot derivatives. Eur J Pharmacol. 1995 Oct 6;293(3):183-90. | ||||
| 703 | Identification of cytochrome P450 enzymes involved in the metabolism of 4'-methoxy-alpha-pyrrolidinopropiophenone (MOPPP), a designer drug, in human liver microsomes. Xenobiotica. 2003 Oct;33(10):989-98. | ||||
| 704 | Preclinical pharmacokinetics of triptolide: a potential antitumor drug. Curr Drug Metab. 2019;20(2):147-154. | ||||
| 705 | In vitro hepatic metabolism of cediranib, a potent vascular endothelial growth factor tyrosine kinase inhibitor: interspecies comparison and human enzymology. Drug Metab Dispos. 2010 Oct;38(10):1688-97. | ||||
| 706 | Involvement of multiple cytochrome P450 and UDP-glucuronosyltransferase enzymes in the in vitro metabolism of muraglitazar. Drug Metab Dispos. 2007 Jan;35(1):139-49. | ||||
| 707 | Characterization of ADME properties of [(14)C]asunaprevir (BMS-650032) in humans. Xenobiotica. 2016;46(1):52-64. | ||||
| 708 | Clinical assessment of gepotidacin (GSK2140944) as a victim and perpetrator of drug-drug interactions via CYP3A metabolism and transporters | ||||
| 709 | FDA label of Sacubitril and valsartan. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 710 | Biotransformation of Finerenone, a Novel Nonsteroidal Mineralocorticoid Receptor Antagonist, in Dogs, Rats, and Humans, In Vivo and In Vitro | ||||
| 711 | Aldosterone and Mineralocorticoid Receptor Signaling as Determinants of Cardiovascular and Renal Injury: From Hans Selye to the Present | ||||
| 712 | Results From Drug-Drug Interaction Studies In Vitro and In Vivo Investigating the Inhibitory Effect of Finerenone on the Drug Transporters BCRP, OATP1B1, and OATP1B3 | ||||
| 713 | Physiologically-based pharmacokinetic modeling to predict CYP3A4-mediated drug-drug interactions of finerenone | ||||
| 714 | The prokinetic cinitapride has no clinically relevant pharmacokinetic interaction and effect on QT during coadministration with ketoconazole. Drug Metab Dispos. 2007 Jul;35(7):1149-56. | ||||
| 715 | Pharmacokinetics, Disposition, and Biotransformation of [(14)C]Omecamtiv Mecarbil in Healthy Male Subjects after a Single Intravenous or Oral Dose | ||||
| 716 | Stereoselective hydroxylation by CYP2C19 and oxidation by ADH4 in the in vitro metabolism of tivantinib | ||||
| 717 | Metabolism and disposition of hepatitis C polymerase inhibitor dasabuvir in humans. Drug Metab Dispos. 2016 Aug;44(8):1139-47. | ||||
| 718 | Metabolic pathway of icotinib in vitro: the differential roles of CYP3A4, CYP3A5, and CYP1A2 on potential pharmacokinetic drug-drug interaction. J Pharm Sci. 2018 Apr;107(4):979-983. | ||||
| 719 | DrugBank(Pharmacology-Metabolism):Chlorpromazine | ||||
| 720 | In vitro and in vivo assessment of the effect of dalcetrapib on a panel of CYP substrates. Curr Med Res Opin. 2009 Apr;25(4):891-902. | ||||
| 721 | Characterization of CYP3A isozymes involved in the metabolism of domperidone: role of cytochrome b5 and inhibition by ketoconazole. Drug Metab Lett. 2010 Apr;4(2):95-103. | ||||
| 722 | Characterization of the metabolism of fenretinide by human liver microsomes, cytochrome P450 enzymes and UDP-glucuronosyltransferases. Br J Pharmacol. 2011 Feb;162(4):989-99. | ||||
| 723 | DrugBank(Pharmacology-Metabolism):Mavacamten | ||||
| 724 | The metabolic profile of azimilide in man: in vivo and in vitro evaluations. J Pharm Sci. 2005 Sep;94(9):2084-95. | ||||
| 725 | DrugBank(Pharmacology-Metabolism):Nestorone | ||||
| 726 | Biosynthesis of all-trans-retinoic acid from all-trans-retinol: catalysis of all-trans-retinol oxidation by human P-450 cytochromes. Drug Metab Dispos. 2000 Mar;28(3):315-22. | ||||
| 727 | Oral laquinimod treatment in multiple sclerosis. Neurologia. 2011 Mar;26(2):111-7. | ||||
| 728 | Studies on the metabolic fate of M17055, a novel diuretic (4): species difference in metabolic pathway and identification of human CYP isoform responsible for the metabolism of M17055 | ||||
| 729 | Metabolism and disposition of the SGLT2 inhibitor bexagliflozin in rats, monkeys and humans | ||||
| 730 | Lack of the effect of lobeglitazone, a peroxisome proliferator-activated receptor-gamma agonist, on the pharmacokinetics and pharmacodynamics of warfarin. Drug Des Devel Ther. 2015 Mar 2;9:737-43. | ||||
| 731 | Human hepatic metabolism of the anti-osteoporosis drug eldecalcitol involves sterol C4-methyl oxidase. Pharmacol Res Perspect. 2015 Mar;3(2):e00120. | ||||
| 732 | Pharmacokinetic evaluation of nabiximols for the treatment of multiple sclerosis pain. Expert Opin Drug Metab Toxicol. 2013 Sep;9(9):1219-28. | ||||
| 733 | Effect of ketoconazole on the pharmacokinetics of maribavir in healthy adults | ||||
| 734 | Metabolism and Disposition of [(14)C]Pevonedistat, a First-in-Class NEDD8-Activating Enzyme Inhibitor, after Intravenous Infusion to Patients with Advanced Solid Tumors | ||||
| 735 | Preclinical metabolism and disposition of SB939 (Pracinostat), an orally active histone deacetylase inhibitor, and prediction of human pharmacokinetics. Drug Metab Dispos. 2011 Dec;39(12):2219-32. | ||||
| 736 | In vitro characterization of the human biotransformation pathways of aplidine, a novel marine anti-cancer drug. Invest New Drugs. 2007 Feb;25(1):9-19. | ||||
| 737 | Profile of blonanserin for the treatment of schizophrenia. Neuropsychiatr Dis Treat. 2013;9:587-94. | ||||
| 738 | Identification of the oxidative and conjugative enzymes involved in the biotransformation of brivanib | ||||
| 739 | Metabolism of 5-methoxypsoralen by Saccharomyces cerevisiae. Photochem Photobiol. 1991 Nov;54(5):689-95. | ||||
| 740 | Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44(8):797-814. | ||||
| 741 | Idalopirdine as a treatment for Alzheimer's disease. Expert Opin Investig Drugs. 2015;24(7):981-7. | ||||
| 742 | Cytochromes P450 2C8 and 3A Catalyze the Metabolic Activation of the Tyrosine Kinase Inhibitor Masitinib | ||||
| 743 | Metabolism and Disposition of Pacritinib (SB1518), an Orally Active Janus Kinase 2 Inhibitor in Preclinical Species and Humans | ||||
| 744 | A Comprehensive Overview of Globally Approved JAK Inhibitors | ||||
| 745 | Absorption, distribution, metabolism, excretion (ADME), drug-drug interaction potential and prediction of human pharmacokinetics of SUVN-G3031, a novel histamine 3 receptor (H(3)R) inverse agonist in clinical development for the treatment of narcolepsy | ||||
| 746 | Disposition and metabolism of the G protein-coupled receptor 40 agonist TAK-875 (fasiglifam) in rats, dogs, and humans. Xenobiotica. 2019 Apr;49(4):433-445. | ||||
| 747 | Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats | ||||
| 748 | Effect of glycyrrhizin on the activity of CYP3A enzyme in humans. Eur J Clin Pharmacol. 2010 Aug;66(8):805-810. | ||||
| 749 | FDA label of Polatuzumab vedotin-piiq. The 2020 official website of the U.S. Food and Drug Administration. | ||||
| 750 | Identification of enzymes responsible for rifalazil metabolism in human liver microsomes. Xenobiotica. 2000 Jun;30(6):565-74. | ||||
| 751 | An integrated assessment of the ADME properties of the CDK4/6 Inhibitor ribociclib utilizing preclinical in vitro, in vivo, and human ADME data | ||||
| 752 | Disposition and metabolism of semagacestat, a {gamma}-secretase inhibitor, in humans. Drug Metab Dispos. 2010 Apr;38(4):554-65. | ||||
| 753 | Prediction of metabolic clearance using fresh human hepatocytes: comparison with cryopreserved hepatocytes and hepatic microsomes for five benzodiazepines. Xenobiotica. 2008 Apr;38(4):353-67. | ||||
| 754 | Complementary pharmacokinetic profiles of netupitant and palonosetron support the rationale for their oral fixed combination for the prevention of chemotherapy-induced nausea and vomiting. J Clin Pharmacol. 2019 Apr;59(4):472-487. | ||||
| 755 | CYP2B6, CYP2D6, and CYP3A4 catalyze the primary oxidative metabolism of perhexiline enantiomers by human liver microsomes. Drug Metab Dispos. 2007 Jan;35(1):128-38. | ||||
| 756 | A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors | ||||
| 757 | Pharmacokinetic profile of dexloxiglumide. Clin Pharmacokinet. 2006;45(12):1177-88. | ||||
| 758 | Characterization of human cytochrome P450 enzymes involved in the biotransformation of eperisone. Xenobiotica. 2009 Jan;39(1):1-10. | ||||
| 759 | Phase I and pharmacological study of a new camptothecin derivative, exatecan mesylate (DX-8951f), infused over 30 minutes every three weeks. Clin Cancer Res. 2001 Oct;7(10):3056-64. | ||||
| 760 | In vitro evaluation of fenfluramine and norfenfluramine as victims of drug interactions | ||||
| 761 | Metabolism and metabolic inhibition of cilnidipine in human liver microsomes. Acta Pharmacol Sin. 2003 Mar;24(3):263-8. | ||||
| 762 | Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44(9):879-94. | ||||
| 763 | Plasma pharmacokinetics and CYP3A12-dependent metabolism of c-kit inhibitor imatinib in dogs | ||||
| 764 | Limitations and off-target effects of tryptophan-related IDO inhibitors in cancer treatment. Front Immunol. 2019 Jul 30;10:1801. | ||||
| 765 | Simultaneous pharmacokinetic model for rolofylline and both M1-trans and M1-cis metabolites. AAPS J. 2013 Apr;15(2):498-504. | ||||
| 766 | Studies on pharmacokinetic drug interaction potential of vinpocetine. Medicines (Basel). 2015 Jun 5;2(2):93-105. | ||||
| 767 | Involvement of cytochrome P450 in the metabolism of rebamipide by the human liver. Xenobiotica. 2002 Jul;32(7):573-86. | ||||
| 768 | Multicenter phase I trial of the mitogen-activated protein kinase 1/2 inhibitor BAY 86-9766 in patients with advanced cancer. Clin Cancer Res. 2013 Mar 1;19(5):1232-43. | ||||
| 769 | Evaluation of in vitro drug interactions with karenitecin, a novel, highly lipophilic camptothecin derivative in phase II clinical development. J Clin Pharmacol. 2003 Sep;43(9):1008-14. | ||||
| 770 | Characterization of the cytochrome P-450 gene family responsible for the N-dealkylation of the ergot alkaloid CQA 206-291 in humans | ||||
| 771 | Effect of grapefruit juice on the disposition of manidipine enantiomers in healthy subjects. Br J Clin Pharmacol. 2006 May;61(5):533-7. | ||||
| 772 | Induction of cytochromes P-450 2B and 3A in mice following the dietary administration of the novel cognitive enhancer linopirdine. Drug Metab Dispos. 1994 Jan-Feb;22(1):65-73. | ||||
| 773 | Ganaxolone | ||||
| 774 | Adverse effects of vitamin E by induction of drug metabolism. Genes Nutr. 2007 Dec;2(3):249-56. | ||||
| 775 | Phase I study of enzastaurin and bevacizumab in patients with advanced cancer: safety, efficacy and pharmacokinetics. Invest New Drugs. 2013 Jun;31(3):653-60. | ||||
| 776 | DrugBank(Pharmacology-Metabolism):Mirvetuximab soravtansine | ||||
| 777 | DrugBank(Pharmacology-Metabolism):MRTX849 | ||||
| 778 | Influence of cytochrome P450 induction on the pharmacokinetics and pharmacodynamics of remacemide hydrochloride. Epilepsy Res. 2002 May;49(3):247-54. | ||||
| 779 | DrugBank(Pharmacology-Metabolism):PT2977 | ||||
| 780 | Effect of ketoconazole on the pharmacokinetics of ornidazole--a possible role of p-glycoprotein and CYP3A. Drug Metabol Drug Interact. 2006;22(1):67-77. | ||||
| 781 | Maribavir pharmacokinetics and the effects of multiple-dose maribavir on cytochrome P450 (CYP) 1A2, CYP 2C9, CYP 2C19, CYP 2D6, CYP 3A, N-acetyltransferase-2, and xanthine oxidase activities in healthy adults. Antimicrob Agents Chemother. 2006 Apr;50(4):1130-5. | ||||
| 782 | Functional Measurement of CYP2C9 and CYP3A4 Allelic Polymorphism on Sildenafil Metabolism | ||||
| 783 | Organic anion transporting polypeptide 1B1 activity classified by SLCO1B1 genotype influences atrasentan pharmacokinetics. Clin Pharmacol Ther. 2006 Mar;79(3):186-96. | ||||
| 784 | Identification of the human liver cytochrome P450 enzymes involved in the in vitro metabolism of a novel 5-lipoxygenase inhibitor. Drug Metab Dispos. 1998 Oct;26(10):970-6. | ||||
| 785 | DrugBank(Pharmacology-Metabolism):Ag-221 | ||||
| 786 | Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018 Sep 19;11(1):120. | ||||
| 787 | Pharmacokinetic effect of AMD070, an Oral CXCR4 antagonist, on CYP3A4 and CYP2D6 substrates midazolam and dextromethorphan in healthy volunteers | ||||
| 788 | Effect of nilvadipine, a dihydropyridine calcium antagonist, on cytochrome P450 activities in human hepatic microsomes. Biol Pharm Bull. 2004 Mar;27(3):415-7. | ||||
| 789 | Amenamevir: studies of potential CYP3A-mediated pharmacokinetic interactions with midazolam, cyclosporine, and ritonavir in healthy volunteers. Clin Pharmacol Drug Dev. 2018 Nov;7(8):844-859. | ||||
| 790 | Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006 Dec;80(6):565-81. | ||||
| 791 | Pharmacokinetics and disposition of momelotinib revealed a disproportionate human metabolite-resolution for clinical development. Drug Metab Dispos. 2018 Mar;46(3):237-247. | ||||
| 792 | Profile of alisporivir and its potential in the treatment of hepatitis C. Drug Des Devel Ther. 2013;7:105-15. | ||||
| 793 | Metabolism of benzalkonium chlorides by human hepatic cytochromes P450. Chem Res Toxicol. 2019 Dec 16;32(12):2466-2478. | ||||
| 794 | Product Information of Eumovate. | ||||
| 795 | Studies of the pharmacology of 17alpha-ethynyl-androst-5-ene-3beta,7beta,17beta-triol, a synthetic anti-inflammatory androstene. Int J Clin Exp Med. 2011;4(2):119-35. | ||||
| 796 | A Pharmacokinetic, Safety, and Tolerability Trial of Palovarotene in Healthy Japanese and Non-Japanese Participants | ||||
| 797 | Accelerating Clinical Development of Idasanutlin through a Physiologically Based Pharmacokinetic Modeling Risk Assessment for CYP450 Isoenzyme-Related Drug-Drug Interactions | ||||
| 798 | The effect of multiple doses of rifampin and ketoconazole on the single-dose pharmacokinetics of ridaforolimus | ||||
| 799 | PubChem:SCY-078 | ||||
| 800 | Metabolism, Excretion, and Mass Balance of Solithromycin in Humans | ||||
| 801 | Oxidative Deamination of Emixustat by Human Vascular Adhesion Protein-1/Semicarbazide-Sensitive Amine Oxidase | ||||
| 802 | Lactobacillus rhamnosus induces CYP3A and changes the pharmacokinetics of verapamil in rats | ||||
| 803 | Effect of verapamil on the pharmacokinetics of hydroxycamptothecin and its potential mechanism | ||||
| 804 | In vitro characterization of sarizotan metabolism: hepatic clearance, identification and characterization of metabolites, drug-metabolizing enzyme identification, and evaluation of cytochrome p450 inhibition. Drug Metab Dispos. 2010 Jun;38(6):905-16. | ||||
| 805 | In vitro and in vivo metabolism and pharmacokinetics of BMS-562086, a potent and orally bioavailable corticotropin-releasing factor-1 receptor antagonist | ||||
| 806 | AMIODARONE AND THYROID DYSFUNCTION | ||||
| 807 | Increased serum amiodarone concentration in hypertriglyceridemic patients: Effects of drug distribution to serum lipoproteins | ||||
| 808 | The contributions of cytochromes P450 3A4 and 3A5 to the metabolism of the phosphodiesterase type 5 inhibitors sildenafil, udenafil, and vardenafil. Drug Metab Dispos. 2008 Jun;36(6):986-90. | ||||
| 809 | Determination of an optimal dosing regimen for fexinidazole, a novel oral drug for the treatment of human African trypanosomiasis: first-in-human studies | ||||
| 810 | Fexinidazole--a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl Trop Dis. 2010 Dec 21;4(12):e923. | ||||
| 811 | Human liver microsomal cytochrome P450 3A isozymes mediated vindesine biotransformation. Metabolic drug interactions. Biochem Pharmacol. 1993 Feb 24;45(4):853-61. | ||||
| 812 | Ramatroban for chemoprophylaxis and treatment of COVID-19: David takes on Goliath | ||||
| 813 | A Physiologically-Based Pharmacokinetic Modeling Approach To Predict Drug-Drug Interactions of Sonidegib (LDE225) with Perpetrators of CYP3A in Cancer Patients | ||||
| 814 | Metabolism, excretion, and pharmacokinetics of ((3,3-difluoropyrrolidin-1-yl)((2S,4S)-4-(4-(pyrimidin-2-yl)piperazin-1-yl)pyrrolidin-2-yl)methanone, a dipeptidyl peptidase inhibitor, in rat, dog and human | ||||
| 815 | Excretion and metabolism of lersivirine (5-{[3,5-diethyl-1-(2-hydroxyethyl)(3,5-14C2)-1H-pyrazol-4-yl]oxy}benzene-1,3-dicarbonitrile), a next-generation non-nucleoside reverse transcriptase inhibitor, after administration of [14C]Lersivirine to healthy volunteers. Drug Metab Dispos. 2010 May;38(5):789-800. | ||||
| 816 | CYP3A but not P-gp plays a relevant role in the in vivo intestinal and hepatic clearance of the delta-specific phosphoinositide-3 kinase inhibitor leniolisib | ||||
| 817 | Minor contribution of CYP3A5 to the metabolism of hepatitis C protease inhibitor paritaprevir in vitro | ||||
| 818 | Main protease inhibitors and drug surface hotspots for the treatment of COVID-19: A drug repurposing and molecular docking approach | ||||
| 819 | Metabolism of carfentanil, an ultra-potent opioid, in human liver microsomes and human hepatocytes by high-resolution mass spectrometry. AAPS J. 2016 Nov;18(6):1489-1499. | ||||
| 820 | Metabolism of the trisubstituted purine cyclin-dependent kinase inhibitor seliciclib (R-roscovitine) in vitro and in vivo. Drug Metab Dispos. 2008 Mar;36(3):561-70. | ||||
| 821 | KAE609 (Cipargamin), a New Spiroindolone Agent for the Treatment of Malaria: Evaluation of the Absorption, Distribution, Metabolism, and Excretion of a Single Oral 300-mg Dose of [14C]KAE609 in Healthy Male Subjects | ||||
| 822 | The Effect of Modafinil on the Safety and Pharmacokinetics of Lorlatinib: A Phase I Study in Healthy Participants | ||||
| 823 | Phenotypic and metabolic investigation of a CSF-1R kinase receptor inhibitor (BLZ945) and its pharmacologically active metabolite | ||||
| 824 | Metabolism and disposition of [14C]BMS-690514 after oral administration to rats, rabbits, and dogs | ||||
| 825 | The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J Hepatol. 2015 Aug;63(2):320-8. | ||||
| 826 | Pharmacokinetics, metabolism, and excretion of licogliflozin, a dual inhibitor of SGLT1/2, in rats, dogs, and humans | ||||
| 827 | Discovery of TD-8954, a clinical stage 5-HT(4) receptor agonist with gastrointestinal prokinetic properties | ||||
| 828 | First-in-human pharmacokinetic and pharmacodynamic study of the dual m-TORC 1/2 inhibitor AZD2014. Clin Cancer Res. 2015 Aug 1;21(15):3412-9. | ||||
| 829 | Characterization of the pharmacokinetics of vilaprisan: bioavailability, excretion, biotransformation, and drug-drug interaction potential. Clin Pharmacokinet. 2018 Aug;57(8):1001-1015. | ||||
| 830 | Evaluation of the Absorption, Metabolism, and Excretion of a Single Oral 1-mg Dose of Tropifexor in Healthy Male Subjects and the Concentration Dependence of Tropifexor Metabolism | ||||
| 831 | Clinical Drug-Drug Interaction Studies to Evaluate the Effects of a P-Glycoprotein Inhibitor, CYP3A Inhibitors, and a CYP3A Inducer on the Pharmacokinetics of Naldemedine in Healthy Subjects | ||||
| 832 | A Population Pharmacokinetic Model of (R)- and (S-) Oxybutynin and Its Active Metabolites After Oral and Intravesical Administration to Healthy Volunteers | ||||
| 833 | Absorption, disposition and metabolic pathway of amiselimod (MT-1303) in healthy volunteers in a mass balance study | ||||
| 834 | Metabolic Profiling of the Novel Hypoxia-Inducible Factor 2 Inhibitor PT2385 In Vivo and In Vitro | ||||
| 835 | Metabolism and disposition of vatalanib (PTK787/ZK-222584) in cancer patients | ||||
| 836 | Vatalanib population pharmacokinetics in patients with myelodysplastic syndrome: CALGB 10105 (Alliance) | ||||
| 837 | Brigatinib: first global approval. Drugs. 2017 Jul;77(10):1131-1135. | ||||
| 838 | DrugBank(Pharmacology-Metabolism):AP26113 | ||||
| 839 | CYP3A4-mediated ester cleavage as the major metabolic pathway of the oral taxane 3'-tert-butyl-3'-N-tert-butyloxycarbonyl-4-deacetyl-3'-dephenyl-3'-N-debenzoyl-4-O-methoxycarbonyl-paclitaxel (BMS-275183) | ||||
| 840 | Quinacrine is mainly metabolized to mono-desethyl quinacrine by CYP3A4/5 and its brain accumulation is limited by P-glycoprotein. Drug Metab Dispos. 2006 Jul;34(7):1136-44. | ||||
| 841 | Physiologically Based Pharmacokinetic Modelling of Inhaled Nemiralisib: Mechanistic Components for Pulmonary Absorption, Systemic Distribution, and Oral Absorption | ||||
| 842 | Integrating Duodenal Sampling in a Human Mass Balance Study to Quantify the Elimination Pathways of JNJ-53718678, a Respiratory Syncytial Virus Fusion Protein Inhibitor | ||||
| 843 | Metabolic characterization of a tripeptide human immunodeficiency virus type 1 protease inhibitor, KNI-272, in rat liver microsomes. Antimicrob Agents Chemother. 1999 Mar;43(3):549-56. | ||||
| 844 | Assessment of the drug interaction risk for remogliflozin etabonate, a sodium-dependent glucose cotransporter-2 inhibitor: evidence from in vitro, human mass balance, and ketoconazole interaction studies | ||||
| 845 | In vitro metabolism of ferroquine (SSR97193) in animal and human hepatic models and antimalarial activity of major metabolites on Plasmodium falciparum. Drug Metab Dispos. 2006 Apr;34(4):667-82. | ||||
| 846 | Frontiers in CNS Drug Discovery. Edited by Atta-ur Rahman, M. Iqbal Choudhary. Page: 318. | ||||
| 847 | Gepirone and 1-(2-pyrimidinyl)-piperazine in vitro: human cytochromes mediating transformation and cytochrome inhibitory effects | ||||
| 848 | 17alpha-alkynyl 3alpha, 17beta-androstanediol non-clinical and clinical pharmacology, pharmacokinetics and metabolism. Invest New Drugs. 2012 Feb;30(1):59-78. | ||||
| 849 | Effect of deuteration on metabolism and clearance of Nerispirdine (HP184) and AVE5638 | ||||
| 850 | Metabolism of the newest antidepressants: comparisons with related predecessors. IDrugs. 2004 Feb;7(2):143-50. | ||||
| 851 | Metabolism and excretion of (S)-6-(3-cyclopentyl-2-(4-trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotinic acid (PF-04991532), a hepatoselective glucokinase activator, in humans: confirmation of the MIST potential noted in first-in-Human metabolite scouting studies | ||||
| 852 | Cytochrome P450 Mediated Bioactivation of Saracatinib | ||||
| 853 | Integration of Physiologically-Based Pharmacokinetic Modeling into Early Clinical Development: An Investigation of the Pharmacokinetic Nonlinearity | ||||
| 854 | In vitro metabolism of CP-122,721 ((2S,3S)-2-phenyl-3-[(5-trifluoromethoxy-2-methoxy)benzylamino]piperidine), a non-peptide antagonist of the substance P receptor. Drug Metab Pharmacokinet. 2007 Oct;22(5):336-49. | ||||
| 855 | Phosphonate O-deethylation of [4-(4-bromo-2-cyano-phenylcarbamoyl) benzyl]-phosphonic acid diethyl ester, a lipoprotein lipase-promoting agent, catalyzed by cytochrome P450 2C8 and 3A4 in human liver microsomes | ||||
| 856 | Evaluation of the inhibitory and induction potential of YM758, a novel If channel inhibitor, for human P450-mediated metabolism | ||||
| 857 | Investigation of the major human hepatic cytochrome P450 involved in 4-hydroxylation and N-dechloroethylation of trofosfamide. Cancer Chemother Pharmacol. 1999;44(4):327-34. | ||||
| 858 | Effect of ketoconazole on the pharmacokinetics of the 11beta-hydroxysteroid dehydrogenase type 1 inhibitor ABT-384 and its two active metabolites in healthy volunteers: population analysis of data from a drug-drug interaction study. Drug Metab Dispos. 2013 May;41(5):1035-45. | ||||
| 859 | Metabolic pathways of the camptothecin analog AR-67 | ||||
| 860 | Metabolism of the MEK1/2 inhibitor pimasertib involves a novel conjugation with phosphoethanolamine in patients with solid tumors. Drug Metab Dispos. 2017 Feb;45(2):174-182. | ||||
| 861 | In vitro assessment of metabolic drugCdrug interaction potential of AZD2624, neurokinin-3 receptor antagonist, through cytochrome P(450) enzyme identification, inhibition, and induction studies | ||||
| 862 | Physiologically based modeling of lisofylline pharmacokinetics following intravenous administration in mice. Eur J Drug Metab Pharmacokinet. 2016 Aug;41(4):403-12. | ||||
| 863 | Identification of human cytochrome P-450 isoforms involved in metabolism of R(+)- and S(-)-gallopamil: utility of in vitro disappearance rate. Drug Metab Dispos. 1999 Nov;27(11):1254-9. | ||||
| 864 | Role of CYP2C9, CYP2C19 and EPHX Polymorphism in the Pharmacokinetic of Phenytoin: A Study on Uruguayan Caucasian Subjects | ||||
| 865 | Metabolic activation of pradefovir by CYP3A4 and its potential as an inhibitor or inducer. Antimicrob Agents Chemother. 2006 Sep;50(9):2926-31. | ||||
| 866 | Pharmacokinetics, tissue distribution and identification of putative metabolites of JI-101 - a novel triple kinase inhibitor in rats | ||||
| 867 | Disposition and metabolism of LY2603618, a Chk-1 inhibitor following intravenous administration in patients with advanced and/or metastatic solid tumors. Xenobiotica. 2014 Sep;44(9):827-41. | ||||
| 868 | The interactions of a selective protein kinase C beta inhibitor with the human cytochromes P450. Drug Metab Dispos. 2002 Sep;30(9):957-61. | ||||
| 869 | Preclinical pharmacokinetics of a HepDirect prodrug of a novel phosphonate-containing thyroid hormone receptor agonist. Drug Metab Dispos. 2008 Nov;36(11):2393-403. | ||||
| 870 | Lack of impact by SCY-078, a first-in-class oral fungicidal glucan synthase inhibitor, on the pharmacokinetics of rosiglitazone, a substrate for CYP450 2C8, supports the low risk for clinically relevant metabolic drug-drug interactions. J Clin Pharmacol. 2018 Oct;58(10):1305-1313. | ||||
| 871 | Ibrexafungerp: A Novel Oral Triterpenoid Antifungal in Development for the Treatment of Candida auris Infections | ||||
| 872 | TB and HIV therapeutics: pharmacology research priorities. AIDS Res Treat. 2012;2012:874083. | ||||
| 873 | Disposition and metabolic profiling of [(14)C]cerlapirdine using accelerator mass spectrometry | ||||
| 874 | Phase I clinical trial of MPC-6827 (Azixa), a microtubule destabilizing agent, in patients with advanced cancer | ||||
| 875 | Effect of human flavin-containing monooxygenase 3 polymorphism on the metabolism of aurora kinase inhibitors | ||||
| 876 | Cytochromes P450 and experimental models of drug metabolism. J Cell Mol Med. 2002 Apr-Jun;6(2):189-98. | ||||
| 877 | Effect of rifampin on the pharmacokinetics, safety and tolerability of navitoclax (ABT-263), a dual inhibitor of Bcl-2 and Bcl-XL , in patients with cancer. J Clin Pharm Ther. 2014 Dec;39(6):680-4. | ||||
| 878 | Physiologically based pharmacokinetic modeling to predict complex drug-drug interactions: a case study of AZD2327 and its metabolite, competitive and time-dependent CYP3A inhibitors | ||||
| 879 | BC-3781: Evaluation of the CYP3A Interaction Potential. | ||||
| 880 | Phase I dose-escalation study of EZN-2208 (PEG-SN38), a novel conjugate of poly(ethylene) glycol and SN38, administered weekly in patients with advanced cancer. Cancer Chemother Pharmacol. 2013 Jun;71(6):1499-506. | ||||
| 881 | The Drug-Drug Interaction Profile of Presatovir | ||||
| 882 | Safety assessment, in vitro and in vivo, and pharmacokinetics of emivirine, a potent and selective nonnucleoside reverse transcriptase inhibitor of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2000 Jan;44(1):123-30. | ||||
| 883 | Cytochrome P450 3A-mediated metabolism of the topoisomerase I inhibitor 9-aminocamptothecin: impact on cancer therapy. Int J Oncol. 2014 Aug;45(2):877-86. | ||||
| 884 | The pharmacogenetics of the new-generation antipsychotics - A scoping review focused on patients with severe psychiatric disorders | ||||
| 885 | Population Pharmacokinetic Model of Oxfendazole and Metabolites in Healthy Adults following Single Ascending Doses | ||||
| 886 | Simulation of human plasma concentration-time profiles of the partial glucokinase activator PF-04937319 and its disproportionate N-demethylated metabolite using humanized chimeric mice and semi-physiological pharmacokinetic modeling | ||||
| 887 | In vitro metabolism, pharmacokinetics and drug interaction potentials of emvododstat, a DHODH inhibitor | ||||
| 888 | A phase 1 healthy male volunteer single escalating dose study of the pharmacokinetics and pharmacodynamics of risdiplam (RG7916, RO7034067), a SMN2 splicing modifier. Br J Clin Pharmacol. 2019 Jan;85(1):181-193. | ||||
| 889 | Comprehensive Medicinal Chemistry III. Page: 569. | ||||
| 890 | Pre-clinical Characterization of Absorption, Distribution, Metabolism and Excretion Properties of TAK-063 | ||||
| 891 | Pharmacokinetics and Safety After a Single Dose of Imarikiren in Subjects with Renal or Hepatic Impairment | ||||
| 892 | Evaluation of the drug-drug interaction potential for trazpiroben (TAK-906), a D(2)/D(3) receptor antagonist for gastroparesis, towards cytochrome P450s and transporters | ||||
| 893 | Population pharmacokinetic modelling of NS2330 (tesofensine) and its major metabolite in patients with Alzheimer's disease | ||||
| 894 | A new compound-specific pleiotropic effect of statins: modification of plasma gamma-tocopherol levels. Atherosclerosis. 2007 Jul;193(1):229-33. | ||||
| 895 | Evaluation of iberdomide and cytochrome p450 drug-drug interaction potential in vitro and in a phase 1 study in healthy subjects | ||||
| 896 | Discovery of Encequidar, First-in-Class Intestine Specific P-glycoprotein Inhibitor | ||||
| 897 | Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes | ||||
| 898 | Review of Clinically Relevant Drug Interactions with Next Generation Hepatitis C Direct-acting Antiviral Agents | ||||
| 899 | Pharmacokinetics and ADME Characterization of Intravenous and Oral [(14)C]-Linerixibat in Healthy Male Volunteers | ||||
| 900 | Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014 Dec;24(12):1670-751. | ||||
| 901 | CYP3A4 and 3A5 catalysed alprazolam metabolism in vitro and in vivo. International Clinical Psychopharmacology, 2006, 21(4):A33. | ||||
| 902 | DrugBank(Pharmacology-Metabolism):KD025 | ||||
| 903 | Identification of human P450 isoforms involved in the metabolism of the antiallergic drug, oxatomide, and its kinetic parameters and inhibition constants. Biol Pharm Bull. 2005 Feb;28(2):328-34. | ||||
| 904 | Elucidation of individual cytochrome P450 enzymes involved in the metabolism of clozapine. Naunyn Schmiedebergs Arch Pharmacol. 1998 Nov;358(5):592-9. | ||||
| 905 | Metabolism and bioactivation of famitinib, a novel inhibitor of receptor tyrosine kinase, in cancer patients. Br J Pharmacol. 2013 Apr;168(7):1687-706. | ||||
| 906 | Pharmacokinetics, safety, and CCR2/CCR5 antagonist activity of cenicriviroc in participants with mild or moderate hepatic impairment. Clin Transl Sci. 2016 Jun;9(3):139-48. | ||||
| 907 | Metabolic map and bioactivation of the anti-tumour drug noscapine | ||||
| 908 | Assessment of drug-drug interaction potential and PBPK modeling of CC-223, a potent inhibitor of the mammalian target of rapamycin kinase. Xenobiotica. 2019 Jan;49(1):54-70. | ||||
| 909 | Biotransformation of tirilazad in human: 1. Cytochrome P450 3A-mediated hydroxylation of tirilazad mesylate in human liver microsomes. J Pharmacol Exp Ther. 1996 May;277(2):982-90. | ||||
| 910 | Pharmacokinetics of ipriflavone and metabolites after oral administration of a corn-oil suspension relative to the Osteofix tablet. Am J Ther. 1997 Jul-Aug;4(7-8):229-38. | ||||
| 911 | Cytotoxicity of chloroacetanilide herbicide alachlor in HepG2 cells independent of CYP3A4 and CYP3A7. Food Chem Toxicol. 2007 May;45(5):871-7. | ||||
| 912 | An in vitro study on the metabolism and possible drug interactions of rokitamycin, a macrolide antibiotic, using human liver microsomes. Drug Metab Dispos. 1999 Jul;27(7):776-85. | ||||
| 913 | The Utilization of Mu-Opioid Receptor Biased Agonists: Oliceridine, an Opioid Analgesic with Reduced Adverse Effects | ||||
| 914 | Main contribution of the cytochrome P450 isoenzyme 1A2 (CYP1A2) to N-demethylation and 5-sulfoxidation of the phenothiazine neuroleptic chlorpromazine in human liver--A comparison with other phenothiazines. Biochem Pharmacol. 2010 Oct 15;80(8):1252-9. | ||||
| 915 | Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naive and previously treated basal cell carcinoma. Clin Cancer Res. 2018 May 1;24(9):2082-2091. | ||||
| 916 | Preclinical metabolism and pharmacokinetics of SB1317 (TG02), a potent CDK/JAK2/FLT3 inhibitor. Drug Metab Lett. 2012 Mar;6(1):33-42. | ||||
| 917 | Drug-metabolizing enzymes and transporters: expression in the human prostate and roles in prostate drug disposition. J Androl. 2006 Mar-Apr;27(2):138-50. | ||||
| 918 | Pharmacokinetics and Drug-Drug Interaction of Ocedurenone (KBP-5074) in vitro and in vivo | ||||
| 919 | Lercanidipine in hypertension. Vasc Health Risk Manag. 2005;1(3):173-82. | ||||
| 920 | Identification of enzymes responsible for primary and sequential oxygenation reactions of capravirine in human liver microsomes. Drug Metab Dispos. 2006 Nov;34(11):1798-802. | ||||
| 921 | In vitro metabolism of irosustat, a novel steroid sulfatase inhibitor: interspecies comparison, metabolite identification, and metabolic enzyme identification | ||||
| 922 | Disposition and metabolism of the cathepsin K inhibitor odanacatib in humans. Drug Metab Dispos. 2014 May;42(5):818-27. | ||||
| 923 | Metabolism and pharmacokinetics of allitinib in cancer patients: the roles of cytochrome P450s and epoxide hydrolase in its biotransformation | ||||
| 924 | Pharmacokinetics and Metabolism of Naringin and Active Metabolite Naringenin in Rats, Dogs, Humans, and the Differences Between Species | ||||
| 925 | Nonclinical pharmacokinetics and in vitro metabolism of H3B-6545, a novel selective ERalpha covalent antagonist (SERCA). Cancer Chemother Pharmacol. 2019 Jan;83(1):151-160. | ||||
| 926 | The wide variability of perospirone metabolism and the effect of perospirone on prolactin in psychiatric patients | ||||
| 927 | Steady-state pharmacokinetics of a new antipsychotic agent perospirone and its active metabolite, and its relationship with prolactin response. Ther Drug Monit. 2004 Aug;26(4):361-5. | ||||
| 928 | Metabolites of PPI-2458, a selective, irreversible inhibitor of methionine aminopeptidase-2: structure determination and in vivo activity. Drug Metab Dispos. 2013 Apr;41(4):814-26. | ||||
| 929 | In vitro metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin in human liver microsomes | ||||
| 930 | In vitro and in vivo clinical pharmacology of dimethyl benzoylphenylurea, a novel oral tubulin-interactive agent | ||||
| 931 | Phase I study of continuous weekly dosing of dimethylamino benzoylphenylurea (BPU) in patients with solid tumours | ||||
| 932 | In Vitro Metabolic Pathways of the New Anti-Diabetic Drug Evogliptin in Human Liver Preparations | ||||
| 933 | Bictegravir, a novel integrase inhibitor for the treatment of HIV infection. Drugs Today (Barc). 2019 Nov;55(11):669-682. | ||||
| 934 | Effect of glimepiride on the pharmacokinetics of teneligliptin in healthy Korean subjects. J Clin Pharm Ther. 2019 Oct;44(5):720-725. | ||||
| 935 | Sex differences in rat hepatic cytolethality of the protein kinase C inhibitor safingol: role of biotransformation. Toxicol Appl Pharmacol. 1996 Apr;137(2):173-81. | ||||
| 936 | Stereoselective disposition of talinolol in man. J Pharm Sci. 2002 Feb;91(2):303-11. | ||||
| 937 | Aldosterone receptor antagonists: effective but often forgotten. Circulation. 2010 Feb 23;121(7):934-9. | ||||
| 938 | Correlation of the in vitro biotransformation of H3B-6527 in dog and human hepatocytes with the in vivo metabolic profile of 14C-H3B-6527 in a dog mass balance study. Xenobiotica. 2020 Apr;50(4):458-467. | ||||
| 939 | MLN8054 and Alisertib (MLN8237): discovery of selective oral aurora A inhibitors. ACS Med Chem Lett. 2015 Apr 22;6(6):630-4. | ||||
| 940 | Metabolic disposition of H3B-8800, an orally available small-molecule splicing modulator, in rats, monkeys, and humans | ||||
| 941 | Unexpected interaction between CYP3A4 and BI 11634: is BI 11634 interacting with CYP3A4 similar to nifedipine? | ||||
| 942 | Metabolism of F18, a derivative of calanolide A, in human liver microsomes and cytosol. Front Pharmacol. 2017 Jul 19;8:479. | ||||
| 943 | Inactivation of cytochrome P450 (P450) 3A4 but not P450 3A5 by OSI-930, a thiophene-containing anticancer drug | ||||
| 944 | MedChemExpress: AZD3839. | ||||
| 945 | Possible interaction between topical terbinafine and acenocoumarol. Ann Pharmacother. 2009 Nov;43(11):1911-2. | ||||
| 946 | ITX 5061 quantitation in human plasma with reverse phase liquid chromatography and mass spectrometry detection. Antivir Ther. 2013;18(3):329-36. | ||||
| 947 | Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability | ||||
| 948 | Management of sugar intolerance in children | ||||
| 949 | Cilomilast: a second generation phosphodiesterase 4 inhibitor for asthma and chronic obstructive pulmonary disease | ||||
| 950 | In vitro metabolism of the novel, highly selective oral angiogenesis inhibitor motesanib diphosphate in preclinical species and in humans | ||||
| 951 | Disposition and metabolism of darapladib, a lipoprotein-associated phospholipase A2 inhibitor, in humans | ||||
| 952 | In vitro and in vivo metabolism of a novel cannabinoid-1 receptor inverse agonist, taranabant, in rats and monkeys | ||||
| 953 | A pharmacokinetic interaction study of avitriptan and propranolol | ||||
| 954 | Metabolism, pharmacokinetics, and protein covalent binding of radiolabeled MaxiPost (BMS-204352) in humans | ||||
| 955 | Absorption, metabolism, and excretion of [14C]MK-0767 (2-methoxy-5-(2,4-dioxo-5-thiazolidinyl)-N-[[4-(trifluoromethyl)phenyl] methyl]benzamide) in humans | ||||
| 956 | Implication of P450-metabolite complex formation in the nonlinear pharmacokinetics and metabolic fate of (+/-)-(1'R*,3R*)-3-phenyl-1-[(1',2',3',4'-tetrahydro-5',6'-methylene-dioxy-1'-naphthalenyl) methyl] pyrrolidine methanesulfonate (ABT-200) in dogs | ||||
| 957 | Metabolism of 1,8-cineole by human cytochrome P450 enzymes: identification of a new hydroxylated metabolite. Biochim Biophys Acta. 2005 Apr 15;1722(3):304-11. doi: 10.1016/j.bbagen.2004.12.019. | ||||
| 958 | Performance comparison between multienzymes loaded single and dual electrodes for the simultaneous electrochemical detection of adenosine and metabolites in cancerous cells. Biosens Bioelectron. 2018 Jun 30;109:263-271. doi: 10.1016/j.bios.2018.03.031. | ||||
| 959 | Identification of the human cytochrome P450s responsible for the in vitro metabolism of a leukotriene B4 receptor antagonist, CP-195,543 | ||||
| 960 | In vitro metabolism of 7 neuronal nicotinic receptor agonist AZD0328 and enzyme identification for its N-oxide metabolite | ||||
| 961 | In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014 Mar 5;383(1-2):181-92. doi: 10.1016/j.mce.2013.12.012. | ||||
| 962 | Main metabolic pathways of TAK-802, a novel drug candidate for voiding dysfunction, in humans: the involvement of carbonyl reduction by 11-hydroxysteroid dehydrogenase 1 | ||||
| 963 | [Investigation of metabolic kinetics and reaction phenotyping of ligustrazin by using liver microsomes and recombinant human enzymes] | ||||
| 964 | Identification of cytochrome P450 isoform involved in the metabolism of YM992, a novel selective serotonin re-uptake inhibitor, in human liver microsomes | ||||
| 965 | Behavior of thymidylate kinase toward monophosphate metabolites and its role in the metabolism of 1-(2'-deoxy-2'-fluoro-beta-L-arabinofuranosyl)-5-methyluracil (Clevudine) and 2',3'-didehydro-2',3'-dideoxythymidine in cells. Antimicrob Agents Chemother. 2005 May;49(5):2044-9. doi: 10.1128/AAC.49.5.2044-2049.2005. | ||||
| 966 | Metabolic mechanism and anti-inflammation effects of sinomenine and its major metabolites N-demethylsinomenine and sinomenine-N-oxide | ||||
| 967 | Combined in vitro-in vivo approach to assess the hepatobiliary disposition of a novel oral thrombin inhibitor | ||||
| 968 | Application of physiologically based pharmacokinetic modeling to understanding the clinical pharmacokinetics of UK-369,003 | ||||
| 969 | Species-dependent metabolism of a novel selective 7 neuronal acetylcholine receptor agonist ABT-107 | ||||
| 970 | In vitro interactions between a potential muscle relaxant E2101 and human cytochromes P450 | ||||
| 971 | In-vitro metabolism of YM17E, an inhibitor of acyl coenzyme A:cholesterol acyltransferase, by liver microsomes in man | ||||
| 972 | Pharmacokinetics, Food Effect, Ketoconazole Interaction, and Safety of JTK-853, a Novel Nonnucleoside HCV Polymerase Inhibitor, After Ascending Single and Multiple Doses in Healthy Subjects | ||||
| 973 | Concurrent induction and mechanism-based inactivation of CYP3A4 by an L-valinamide derivative | ||||
| 974 | Metabolism of ezlopitant, a nonpeptidic substance P receptor antagonist, in liver microsomes: enzyme kinetics, cytochrome P450 isoform identity, and in vitro-in vivo correlation. Drug Metab Dispos. 2000 Sep;28(9):1069-76. | ||||
| 975 | Pharmacokinetics, metabolism, and excretion of torcetrapib, a cholesteryl ester transfer protein inhibitor, in humans. Drug Metab Dispos. 2008 Nov;36(11):2185-98. | ||||
| 976 | Identification of cytochrome P4503A as the major subfamily responsible for the metabolism of roquinimex in man. Xenobiotica. 2000 Sep;30(9):905-14. | ||||
| 977 | In vitro evaluation of potential in vivo probes for human flavin-containing monooxygenase (FMO): metabolism of benzydamine and caffeine by FMO and P450 isoforms. Br J Clin Pharmacol. 2000 Oct;50(4):311-4. | ||||
| 978 | In vitro metabolism studies of nomifensine monooxygenation pathways: metabolite identification, reaction phenotyping, and bioactivation mechanism | ||||
| 979 | Identification of cytochromes P450 involved in the human liver microsomal metabolism of the thromboxane A2 inhibitor seratrodast (ABT-001). Drug Metab Dispos. 1997 Jan;25(1):110-5. | ||||
| 980 | Pharmacokinetic assessment of the sites of first-pass metabolism of BMS-181101, an antidepressant agent, in rats. J Pharm Pharmacol. 1998 Mar;50(3):275-8. | ||||
| 981 | Cytochrome P450 Involvement in the biotransformation of cisapride and racemic norcisapride in vitro: differential activity of individual human CYP3A isoforms. Drug Metab Dispos. 2001 Dec;29(12):1548-54. | ||||
| 982 | Metabolism of mofarotene in hepatocytes and liver microsomes from different species. Comparison with in vivo data and evaluation of the cytochrome P450 isoenzymes involved in human biotransformation. Drug Metab Dispos. 1995 Oct;23(10):1051-7. | ||||
| 983 | Bioreductive GDEPT using cytochrome P450 3A4 in combination with AQ4N. Cancer Gene Ther. 2003 Jan;10(1):40-8. | ||||
| 984 | Antiviral activity, pharmacokinetics, and safety of BMS-488043, a novel oral small-molecule HIV-1 attachment inhibitor, in HIV-1-infected subjects. Antimicrob Agents Chemother. 2011 Feb;55(2):722-8. | ||||
| 985 | Metabolism of vanoxerine, 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine, by human cytochrome P450 enzymes. Drug Metab Dispos. 2001 Sep;29(9):1216-20. | ||||
| 986 | Hepatotoxicity observed in clinical trials of aplaviroc (GW873140). Antimicrob Agents Chemother. 2008 Mar;52(3):858-65. | ||||
| 987 | Induction of CYP3As in HepG2 cells by several drugs. Association between induction of CYP3A4 and expression of glucocorticoid receptor. Biol Pharm Bull. 2003 Apr;26(4):510-7. | ||||
| 988 | GW766994 CCR3 receptor antagonist. | ||||
| 989 | The mechanism of aphidicolin bioinactivation by rat liver in vitro systems. Xenobiotica. 1990 Mar;20(3):273-87. | ||||
| 990 | Seville (sour) orange juice: synephrine content and cardiovascular effects in normotensive adults. J Clin Pharmacol. 2001 Oct;41(10):1059-63. | ||||
| 991 | Stereoselective pharmacokinetics of RS-8359, a selective and reversible MAO-A inhibitor, by species-dependent drug-metabolizing enzymes. Chirality. 2005 Mar;17(3):135-41. | ||||
| 992 | Effects of tesmilifene, a substrate of CYP3A and an inhibitor of P-glycoprotein, on the pharmacokinetics of intravenous and oral docetaxel in rats. J Pharm Pharmacol. 2010 Aug;62(8):1084-8. | ||||
| 993 | Using chimeric mice with humanized livers to predict human drug metabolism and a drug-drug interaction. J Pharmacol Exp Ther. 2013 Feb;344(2):388-96. | ||||
| 994 | The critical role of oxidative stress in the toxicity and metabolism of quinoxaline 1,4-di-N-oxides in vitro and in vivo. Drug Metab Rev. 2016 May;48(2):159-82. | ||||
| 995 | Identification of the human P450 enzymes involved in the in vitro metabolism of the synthetic steroidal hormones Org 4060 and Org 30659. Xenobiotica. 2002 Feb;32(2):109-18. | ||||
| 996 | Identification of the cytochromes P450 that catalyze coumarin 3,4-epoxidation and 3-hydroxylation | ||||
| 997 | Metabolic activation of aromatic amines by human pancreas. Carcinogenesis. 1997 May;18(5):1085-92. | ||||
| 998 | CYP3A4 and CYP2A6 activities marked by the metabolism of lignocaine and coumarin in patients with liver and kidney diseases and epileptic patients. Br J Clin Pharmacol. 1995 Jan;39(1):71-6. | ||||
| 999 | Metabolism of butyrylfentanyl in fresh human hepatocytes: chemical synthesis of authentic metabolite standards for definitive identification. Biol Pharm Bull. 2019;42(4):623-630. | ||||
| 1000 | Kinetic characterization and identification of the enzymes responsible for the hepatic biotransformation of adinazolam and N-desmethyladinazolam in man. J Pharm Pharmacol. 1998 Mar;50(3):265-74. | ||||
| 1001 | Midazolam and other benzodiazepines. Handb Exp Pharmacol. 2008;(182):335-60. | ||||
| 1002 | In vitro drug allergy detection system incorporating human liver microsomes in chlorazepate-induced skin rash: drug-specific proliferation associated with interleukin-5 secretion. Br J Dermatol. 2001 Feb;144(2):316-20. | ||||
| 1003 | Involvement of multiple human cytochromes P450 in the liver microsomal metabolism of astemizole and a comparison with terfenadine. Br J Clin Pharmacol. 2001 Feb;51(2):133-42. | ||||
| 1004 | Major pathway of imipramine metabolism is catalyzed by cytochromes P-450 1A2 and P-450 3A4 in human liver. Mol Pharmacol. 1993 May;43(5):827-32. | ||||
| 1005 | Cytochrome P450 4A11 inhibition assays based on characterization of lauric acid metabolites | ||||
| 1006 | Characterization of human cytochrome P450 enzymes involved in the metabolism of cyamemazine. Eur J Pharm Sci. 2007 Dec;32(4-5):357-66. | ||||
| 1007 | The naphthol selective estrogen receptor modulator (SERM), LY2066948, is oxidized to an o-quinone analogous to the naphthol equine estrogen, equilenin. Chem Biol Interact. 2012 Mar 5;196(1-2):1-10. | ||||
| 1008 | Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver. Toxicology. 2006 Jul 5;224(1-2):22-32. | ||||
| 1009 | 6alpha-hydroxylation of taurochenodeoxycholic acid and lithocholic acid by CYP3A4 in human liver microsomes. Biochim Biophys Acta. 1999 Apr 19;1438(1):47-54. | ||||
| 1010 | Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci U S A. 2010 Oct 26;107(43):18422-7. | ||||
| 1011 | Diet Drug Porfimer sodium Safe, Effective, Study Finds. | ||||
| 1012 | Cytochrome P450 responsible for the stereoselective S-oxidation of flosequinan in hepatic microsomes from rats and humans. Drug Metab Dispos. 1997 Jun;25(6):716-24. | ||||
| 1013 | [Trazodon--the antidepressant: mechanism of action and its position in the treatment of depression]. Psychiatr Pol. 2011 Jul-Aug;45(4):611-25. | ||||
| 1014 | Metabolism of medroxyprogesterone acetate (MPA) via CYP enzymes in vitro and effect of MPA on bleeding time in female rats in dependence on CYP activity in vivo. Life Sci. 2003 Nov 7;73(25):3201-12. | ||||
| 1015 | Inhibition of triazolam clearance by macrolide antimicrobial agents: in vitro correlates and dynamic consequences. Clin Pharmacol Ther. 1998 Sep;64(3):278-85. | ||||
| 1016 | Human liver oxidative metabolism of O6-benzylguanine. Biochem Pharmacol. 1995 Oct 26;50(9):1385-9. | ||||
| 1017 | P450 enzyme expression patterns in the NCI human tumor cell line panel. Drug Metab Dispos. 2001 Mar;29(3):304-12. | ||||
| 1018 | CYP3A time-dependent inhibition risk assessment validated with 400 reference drugs. Drug Metab Dispos. 2011 Jun;39(6):1039-46. | ||||
| 1019 | Metabolism of JQ1, an inhibitor of bromodomain and extra terminal bromodomain proteins, in human and mouse liver microsomes? | ||||
| 1020 | New insights in the metabolism of oxybutynin: evidence of N-oxidation of propargylamine moiety and rearrangement to enaminoketone | ||||
| 1021 | Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer Nature. 2015 Jul 16;523(7560):347-51. doi: 10.1038/nature14406. | ||||
| 1022 | Disposition of asciminib, a potent BCR-ABL1 tyrosine kinase inhibitor, in healthy male subjects | ||||
| 1023 | High-performance liquid chromatographic determination of seratrodast and its metabolites in human serum and urine | ||||
| 1024 | Metabolism and Disposition of the Hepatitis C Protease Inhibitor Paritaprevir in Humans | ||||
| 1025 | Bioavailability, Biotransformation, and Excretion of the Covalent Bruton Tyrosine Kinase Inhibitor Acalabrutinib in Rats, Dogs, and Humans Drug Metab Dispos. 2019 Feb;47(2):145-154. doi: 10.1124/dmd.118.084459. | ||||
| 1026 | Identification and Characterization of ACP-5862, the Major Circulating Active Metabolite of Acalabrutinib: Both Are Potent and Selective Covalent Bruton Tyrosine Kinase Inhibitors?. J Pharmacol Exp Ther. 2023 Jan;384(1):173-186. doi: 10.1124/jpet.122.001116. | ||||
| 1027 | Bioequivalence and Relative Bioavailability Studies to Assess a New Acalabrutinib Formulation That Enables Coadministration With Proton-Pump Inhibitors. Clin Pharmacol Drug Dev. 2022 Nov;11(11):1294-1307. doi: 10.1002/cpdd.1153. | ||||
| 1028 | Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents FASEB J. 2010 Dec;24(12):4904-16. doi: 10.1096/fj.10-162438. | ||||
| 1029 | Rapid detection and quantification of paracetamol and its major metabolites using surface enhanced Raman scattering. Analyst. 2023 Apr 11;148(8):1805-1814. doi: 10.1039/d3an00249g. | ||||
| 1030 | Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis B J Med Chem. 2008 Feb 14;51(3):666-76. doi: 10.1021/jm7012216. | ||||
| 1031 | Nanocrystal Suspensions for Enhancing the Oral Absorption of Albendazole. Nanomaterials (Basel). 2022 Sep 1;12(17):3032. doi: 10.3390/nano12173032. | ||||
| 1032 | Multi-residue methodology for quantification of antiparasitics in hen eggs by LC-MS/MS: development, validation and application to 348 samples from Brazil. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2022 Aug;39(8):1412-1423. doi: 10.1080/19440049.2022.2093984. | ||||
| 1033 | Improvement of Albendazole Bioavailability with Menbutone Administration in Sheep. Animals (Basel). 2022 Feb 14;12(4):463. doi: 10.3390/ani12040463. | ||||
| 1034 | Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY:McGraw-Hill, 2001., p. 1126 | ||||
| 1035 | Effect of clotrimazole on microsomal metabolism and pharmacokinetics of albendazole J Pharm Pharmacol. 2003 Jun;55(6):757-64. doi: 10.1211/002235703765951357. | ||||
| 1036 | Metabolism of alfentanil by cytochrome p4503a (cyp3a) enzymes. Drug Metab Dispos. 2005 Mar;33(3):303-11. | ||||
| 1037 | Alfentanil pharmacokinetics and metabolism in humans Anesthesiology. 1988 Oct;69(4):527-34. doi: 10.1097/00000542-198810000-00012. | ||||
| 1038 | LABEL:LFUZOSIN HYDROCHLORIDE EXTENDED RELEASE- alfuzosin hydrochloride tablet | ||||
| 1039 | Absorption, distribution, metabolism, and elimination of the direct renin inhibitor aliskiren in healthy volunteers Drug Metab Dispos. 2007 Aug;35(8):1418-28. doi: 10.1124/dmd.106.013797. | ||||
| 1040 | DrugBank(Pharmacology-Metabolism):Almogran | ||||
| 1041 | Absorption, distribution, metabolism, and excretion of [(14)C]BYL719 (alpelisib) in healthy male volunteers. Cancer Chemother Pharmacol. 2015 Oct;76(4):751-60. | ||||
| 1042 | Alprazolam metabolism in vitro: studies of human, monkey, mouse, and rat liver microsomes | ||||
| 1043 | Drug metabolism in human brain: high levels of cytochrome P4503A43 in brain and metabolism of anti-anxiety drug alprazolam to its active metabolite | ||||
| 1044 | Effect of ketoconazole on the pharmacokinetic profile of ambrisentan. J Clin Pharmacol. 2009 Jun;49(6):719-24. | ||||
| 1045 | Potential for pharmacokinetic interactions between ambrisentan and cyclosporine. Clin Pharmacol Ther. 2010 Oct;88(4):513-20. | ||||
| 1046 | Determination of the cardiac drug amiodarone and its N-desethyl metabolite in sludge samples | ||||
| 1047 | Amiodarone and concurrent antiretroviral therapy: a case report and review of the literature | ||||
| 1048 | Oxidative metabolism of amprenavir in the human liver. Effect of the CYP3A maturation. Drug Metab Dispos. 2003 Mar;31(3):275-81. | ||||
| 1049 | Effects of cytokine-induced macrophages on the response of tumor cells to banoxantrone (AQ4N) | ||||
| 1050 | Bioreductively activated antitumor N-oxides: the case of AQ4N, a unique approach to hypoxia-activated cancer chemotherapy | ||||
| 1051 | CYP2S1 is negatively regulated by corticosteroids in human cell lines. Toxicol Lett. 2012 Feb 25;209(1):30-4. | ||||
| 1052 | Apalutamide Absorption, Metabolism, and Excretion in Healthy Men, and Enzyme Reaction in Human Hepatocytes Drug Metab Dispos. 2019 May;47(5):453-464. doi: 10.1124/dmd.118.084517. | ||||
| 1053 | Apixaban. Hosp Pharm. 2013 Jun;48(6):494-509. | ||||
| 1054 | Apixaban. After hip or knee replacement: LMWH remains the standard treatment. Prescrire Int. 2012 Sep;21(130):201-2, 204. | ||||
| 1055 | Disposition, metabolism and mass balance of [(14)C]apremilast following oral administration. Xenobiotica. 2011 Dec;41(12):1063-75. | ||||
| 1056 | Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos. 2004 Nov;32(11):1287-92. | ||||
| 1057 | Genetic toxicology and toxicokinetics of arecoline and related areca nut compounds: an updated review | ||||
| 1058 | Aripiprazole Lauroxil: Pharmacokinetic Profile of This Long-Acting Injectable Antipsychotic in Persons With Schizophrenia J Clin Psychopharmacol. 2017 Jun;37(3):289-295. doi: 10.1097/JCP.0000000000000691. | ||||
| 1059 | Biological conversion of?aripiprazole lauroxil - An N-acyloxymethyl aripiprazole prodrug Results Pharma Sci. 2014 May 2;4:19-25. doi: 10.1016/j.rinphs.2014.04.002. | ||||
| 1060 | LABEL:RMODAFINIL tablet | ||||
| 1061 | Synthesis, characterization, and antimalarial activity of the glucuronides of the hydroxylated metabolites of arteether | ||||
| 1062 | Use of a physiologically-based pharmacokinetic model to simulate artemether dose adjustment for overcoming the drug-drug interaction with efavirenz In Silico Pharmacol. 2013 Mar 1;1:4. doi: 10.1186/2193-9616-1-4. | ||||
| 1063 | CYP3A-mediated generation of aldehyde and hydrazine in atazanavir metabolism Drug Metab Dispos. 2011 Mar;39(3):394-401. doi: 10.1124/dmd.110.036327. | ||||
| 1064 | Influence of Drug-Drug Interactions on the Pharmacokinetics of Atorvastatin and Its Major Active Metabolite ortho-OH-Atorvastatin in Aging People Living with HIV | ||||
| 1065 | Development of an LC-MS/MS method for simultaneous determination of ticagrelor and its active metabolite during concomitant treatment with atorvastatin | ||||
| 1066 | Rifampin markedly decreases and gemfibrozil increases the plasma concentrations of atorvastatin and its metabolites | ||||
| 1067 | LC-MS/MS determination of avanafil and its metabolites in rat plasma and brain: pharmacokinetic study after oral administration and transdermal film application | ||||
| 1068 | FDA:Avanafil | ||||
| 1069 | In Vitro Kinetic Characterization of Axitinib Metabolism | ||||
| 1070 | Vinpocetine, cognition, and epilepsy | ||||
| 1071 | Phase I study of orally administered (14)Carbon-isotope labelled-vistusertib (AZD2014), a dual TORC1/2 kinase inhibitor, to assess the absorption, metabolism, excretion, and pharmacokinetics in patients with advanced solid malignancies | ||||
| 1072 | Metabolic disposition of AZD8931, an oral equipotent inhibitor of EGFR, HER2 and HER3 signalling, in rat, dog and man | ||||
| 1073 | BC-3781:Evaluation of the CYP3A Interaction Potential. | ||||
| 1074 | Effect of CYP3A5*3 genetic variant on the metabolism of direct-acting antivirals in vitro: a different effect on asunaprevir versus daclatasvir and beclabuvir J Hum Genet. 2020 Jan;65(2):143-153. doi: 10.1038/s10038-019-0685-2. | ||||
| 1075 | DrugBank(Pharmacology-Metabolism):Beclomethasone dipropionate | ||||
| 1076 | Effects of Rifamycin Coadministration on Bedaquiline Desmethylation in Healthy Adult Volunteers | ||||
| 1077 | Pharmacokinetics, metabolism, and excretion of (14)C-labeled belinostat in patients with recurrent or progressive malignancies | ||||
| 1078 | Identification of Epoxide-Derived Metabolite(s) of Benzbromarone | ||||
| 1079 | The metabolism of berberine and its contribution to the pharmacological effects | ||||
| 1080 | LABEL: JUXTAPID- lomitapide mesylate capsule | ||||
| 1081 | Glucuronidation as a major metabolic clearance pathway of 14c-labeled muraglitazar in humans: metabolic profiles in subjects with or without bile collection Drug Metab Dispos. 2006 Mar;34(3):427-39. doi: 10.1124/dmd.105.007617. | ||||
| 1082 | Utilization of in vitro Caco-2 permeability and liver microsomal half-life screens in discovering BMS-488043, a novel HIV-1 attachment inhibitor with improved pharmacokinetic properties | ||||
| 1083 | DrugBank(Pharmacology-Metabolism)Asunaprevir | ||||
| 1084 | Metabolism and disposition of [14C]BMS-690514, an ErbB/vascular endothelial growth factor receptor inhibitor, after oral administration to humans | ||||
| 1085 | U. S. FDA Label -BNP-1350 | ||||
| 1086 | Characterization of human liver enzymes involved in the biotransformation of boceprevir, a hepatitis C virus protease inhibitor | ||||
| 1087 | Physiologically-Based Pharmacokinetic Predictions of the Effect of Curcumin on Metabolism of Imatinib and Bosutinib: In Vitro and In Vivo Disconnect | ||||
| 1088 | DrugBank(Pharmacology-Metabolism)Brentuximab vedotin | ||||
| 1089 | Pharmacokinetics and metabolism of brexpiprazole, a novel serotonin-dopamine activity modulator and its main metabolite in rat, monkey and human. Xenobiotica. 2021 May;51(5):590-604. doi: 10.1080/00498254.2021.1890275. | ||||
| 1090 | Pharmacokinetics and metabolism of 14C-brivaracetam, a novel SV2A ligand, in healthy subjects. Drug Metab Dispos. 2008 Jan;36(1):36-45. | ||||
| 1091 | [Synthesis of Butyl 6alpha-Fluoro-11beta-hydroxy-16alpha-methyl-3,20-dioxo-1,4-pregnadien-21-oate (Fluocortin Butylester) (author's transl)] | ||||
| 1092 | Metabolite profiling and reaction phenotyping for the in vitro assessment of the bioactivation of bromfenac. Chem Res Toxicol. 2020 Jan 21;33(1):249-257. | ||||
| 1093 | Metabolic disposition of 14C-bromfenac in healthy male volunteers J Clin Pharmacol. 1998 Aug;38(8):744-52. doi: 10.1002/j.1552-4604.1998.tb04815.x. | ||||
| 1094 | Fate and disposition of bromocriptine in animals and man. II: Absorption, elimination and metabolism | ||||
| 1095 | Fate and disposition of bromocriptine in animals and man. I: structure elucidation of the metabolites | ||||
| 1096 | Contribution of cytochrome P450 3A pathway to bromocriptine metabolism and effects of ferrous iron and hypoxia-re-oxygenation on its elimination in the perfused rat liver | ||||
| 1097 | Structural elucidation of phase I and II metabolites of bupivacaine in horse urine and fungi of the Cunninghamella species using liquid chromatography/multi-stage mass spectrometry Rapid Commun Mass Spectrom. 2012 Jun 15;26(11):1338-46. doi: 10.1002/rcm.6225. | ||||
| 1098 | In vitro metabolism study of buprenorphine: evidence for new metabolic pathways. Drug Metab Dispos. 2005 May;33(5):689-95. | ||||
| 1099 | DrugBank(Pharmacology-Metabolism)Buspirone | ||||
| 1100 | Metabolism and disposition of buspirone Am J Med. 1986 Mar 31;80(3B):41-51. doi: 10.1016/0002-9343(86)90331-1. | ||||
| 1101 | Cytochrome P450 3A-mediated metabolism of buspirone in human liver microsomes. Drug Metab Dispos. 2005 Apr;33(4):500-7. | ||||
| 1102 | Interaction of buspirone and its major metabolites with human organic cation transporters. Fundam Clin Pharmacol. 2023 Feb 26. doi: 10.1111/fcp.12883. | ||||
| 1103 | A phase I open-label study investigating the disposition of [14C]-cabazitaxel in patients with advanced solid tumors Anticancer Drugs. 2015 Mar;26(3):350-8. doi: 10.1097/CAD.0000000000000185. | ||||
| 1104 | Clinical pharmacokinetics of cabergoline Clin Pharmacokinet. 2003;42(7):633-45. doi: 10.2165/00003088-200342070-00003. | ||||
| 1105 | Clinical Pharmacokinetics and Pharmacodynamics of Cabozantinib Clin Pharmacokinet. 2017 May;56(5):477-491. doi: 10.1007/s40262-016-0461-9. | ||||
| 1106 | PharmGKB summary: caffeine pathway. Pharmacogenet Genomics. 2012 May;22(5):389-95. | ||||
| 1107 | Urine caffeine metabolites are positively associated with cognitive performance in older adults: An analysis of US National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Nutr Res. 2023 Jan;109:12-25. doi: 10.1016/j.nutres.2022.11.002. | ||||
| 1108 | Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011 Jan;1814(1):186-99. | ||||
| 1109 | Clinical Pharmacokinetic, Pharmacodynamic, and Drug-Drug Interaction Profile of Canagliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor Clin Pharmacokinet. 2015 Oct;54(10):1027-41. doi: 10.1007/s40262-015-0285-z. | ||||
| 1110 | Carbamazepine and its metabolites in human perfused placenta and in maternal and cord blood Epilepsia. 1995 Mar;36(3):241-8. doi: 10.1111/j.1528-1157.1995.tb00991.x. | ||||
| 1111 | Metabolism of carvedilol in dogs, rats, and mice Drug Metab Dispos. 1998 Oct;26(10):958-69. | ||||
| 1112 | CYP3A5 mediates bioactivation and cytotoxicity of tetrandrine. Arch Toxicol. 2016 Jul;90(7):1737-48. | ||||
| 1113 | Antiserotonergic properties of terguride in blood vessels, platelets, and valvular interstitial cells | ||||
| 1114 | Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012 Apr;22(4):310-8. | ||||
| 1115 | DrugBank(Pharmacology-Metabolism)Cerivastatin | ||||
| 1116 | Identification of human drug-metabolizing enzymes involved in the metabolism of SNI-2011. Biol Pharm Bull. 2001 Nov;24(11):1263-6. | ||||
| 1117 | Chloramphenicol is a potent inhibitor of cytochrome P450 isoforms CYP2C19 and CYP3A4 in human liver microsomes. Antimicrob Agents Chemother. 2003 Nov;47(11):3464-9. | ||||
| 1118 | Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements Clin Pharmacokinet. 1996 Oct;31(4):257-74. doi: 10.2165/00003088-199631040-00003. | ||||
| 1119 | Cytochrome P450 inactivation by pharmaceuticals and phytochemicals: therapeutic relevance Drug Metab Rev. 2008;40(1):101-47. doi: 10.1080/03602530701836704. | ||||
| 1120 | Classics in Chemical Neuroscience: Chlorpromazine ACS Chem Neurosci. 2019 Jan 16;10(1):79-88. doi: 10.1021/acschemneuro.8b00258. | ||||
| 1121 | Determination of chlorpromazine and its metabolites in animal-derived foods using QuEChERS-based extraction, EMR-Lipid cleanup, and UHPLC-Q-Orbitrap MS analysis. Food Chem. 2023 Mar 1;403:134298. doi: 10.1016/j.foodchem.2022.134298. | ||||
| 1122 | Genotyping studies of Toxoplasma gondii isolates from Africa revealed that the archetypal clonal lineages predominate as in North America and Europe Vet Parasitol. 2008 Aug 17;155(3-4):314-8. doi: 10.1016/j.vetpar.2008.04.021. | ||||
| 1123 | In Vitro metabolism of ciclesonide in human lung and liver precision-cut tissue slices Biopharm Drug Dispos. 2006 May;27(4):197-207. doi: 10.1002/bdd.500. | ||||
| 1124 | Cyclosporin metabolism in transplant patients | ||||
| 1125 | Determination of cilostazol and its metabolites in human urine by high performance liquid chromatography J Pharm Biomed Anal. 2001 Jan;24(3):381-9. doi: 10.1016/s0731-7085(00)00426-x. | ||||
| 1126 | Characterization of human cytochrome p450 enzymes involved in the metabolism of cilostazol Drug Metab Dispos. 2007 Oct;35(10):1730-2. doi: 10.1124/dmd.107.016758. | ||||
| 1127 | Tamoxifen dose and serum concentrations of tamoxifen and six of its metabolites in routine clinical outpatient care | ||||
| 1128 | Identification of the cytochrome P450 enzymes involved in the metabolism of cisapride: in vitro studies of potential co-medication interactions. Br J Pharmacol. 2000 Apr;129(8):1655-67. | ||||
| 1129 | Analytical methodologies for the enantiodetermination of citalopram and its metabolites | ||||
| 1130 | Metabolism, pharmacokinetics, and excretion of a nonpeptidic substance P receptor antagonist, ezlopitant, in normal healthy male volunteers: characterization of polar metabolites by chemical derivatization with dansyl chloride | ||||
| 1131 | Aquatic toxicity of the macrolide antibiotic clarithromycin and its metabolites | ||||
| 1132 | Kinetics and metabolism of clobazam in animals and man | ||||
| 1133 | Characterization of Clofazimine Metabolism in Human Liver Microsomal Incubation In Vitro | ||||
| 1134 | Metabolism of clomipramine in a Japanese psychiatric population: hydroxylation, desmethylation, and glucuronidation | ||||
| 1135 | Population pharmacokinetics of clomipramine, desmethylclomipramine, and hydroxylated metabolites in patients with depression receiving chronic treatment: model evaluation | ||||
| 1136 | Optimization of Clonazepam Therapy Adjusted to Patient's CYP3A Status and NAT2 Genotype | ||||
| 1137 | Determination of clorazepate and its major metabolites in blood and urine by electron capture gas-liquid chromatography | ||||
| 1138 | [Interest in learning (2)] | ||||
| 1139 | Biotransformation of cobicistat: metabolic pathways and enzymes. Drug Metab Lett. 2016;10(2):111-23. | ||||
| 1140 | Liquid chromatography--tandem mass spectrometry analysis of cocaine and its metabolites from blood, amniotic fluid, placental and fetal tissues: study of the metabolism and distribution of cocaine in pregnant rats | ||||
| 1141 | Pharmacogenetics of analgesic drugs | ||||
| 1142 | Colchicine-antimicrobial drug interactions: what pharmacists need to know in treating gout | ||||
| 1143 | U. S. FDA Label -Conivaptan hydrochloride | ||||
| 1144 | Conjugated estrogens and bazedoxifene | ||||
| 1145 | Effect of azole antifungals ketoconazole and fluconazole on the pharmacokinetics of dexloxiglumide. Br J Clin Pharmacol. 2005 Nov;60(5):498-507. | ||||
| 1146 | Active cyamemazine metabolites in patients treated with cyamemazine (Tercian?): influence on cerebral dopamine D2 and serotonin 5-HT (2A) receptor occupancy as measured by positron emission tomography (PET) | ||||
| 1147 | The KEGG resource for deciphering the genome | ||||
| 1148 | U. S. FDA Label -Cyclobenzaprine | ||||
| 1149 | Aprepitant inhibits cyclophosphamide bioactivation and thiotepa metabolism | ||||
| 1150 | Uptake, Elimination, and Biotransformation Potential of a Progestagen (Cyproterone Acetate) in Tilapia Exposed at an Environmental Concentration | ||||
| 1151 | Metabolism and disposition of oral dabrafenib in cancer patients: proposed participation of aryl nitrogen in carbon-carbon bond cleavage via decarboxylation following enzymatic oxidation | ||||
| 1152 | In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans Drug Metab Dispos. 2010 Mar;38(3):405-14. doi: 10.1124/dmd.109.029165. | ||||
| 1153 | Dapoxetine: a new option in the medical management of premature ejaculation. Ther Adv Urol. 2012 Oct;4(5):233-51. | ||||
| 1154 | Effects of Evodiamine on the Pharmacokinetics of Dapoxetine and Its Metabolite Desmethyl Dapoxetine in Rats | ||||
| 1155 | Effects of 22 novel CYP2D6 variants found in Chinese population on the metabolism of dapoxetine | ||||
| 1156 | LC-Q-TOF-MS/MS determination of darunavir and its metabolites in rat serum and urine: application to pharmacokinetics J Pharm Biomed Anal. 2014 Jun;94:92-8. doi: 10.1016/j.jpba.2014.01.035. | ||||
| 1157 | Identification of the human enzymes involved in the oxidative metabolism of dasatinib: an effective approach for determining metabolite formation kinetics. Drug Metab Dispos. 2008 Sep;36(9):1828-39. | ||||
| 1158 | Force degradation behavior of glucocorticoid deflazacort by UPLC: isolation, identification and characterization of degradant by FTIR, NMR and mass analysis J Biomed Res. 2016 Mar;30(2):149-161. doi: 10.7555/JBR.30.20150074. | ||||
| 1159 | Resveratrol Inhibits Key Steps of Steroid Metabolism in a Human Estrogen-Receptor Positive Breast Cancer Model: Impact on Cellular Proliferation Front Pharmacol. 2018 Jul 10;9:742. doi: 10.3389/fphar.2018.00742. | ||||
| 1160 | Identification of the metabolites of the HIV-1 reverse transcriptase inhibitor delavirdine in monkeys Drug Metab Dispos. 1997 Jul;25(7):814-27. | ||||
| 1161 | DrugBank(Pharmacology-Metabolism):Desvenlafaxine | ||||
| 1162 | DrugBank(Pharmacology-Metabolism)Desvenlafaxine succinate | ||||
| 1163 | Dexamethasone metabolism in vitro: species differences J Steroid Biochem Mol Biol. 1997 Jul;62(4):345-52. doi: 10.1016/s0960-0760(97)00038-1. | ||||
| 1164 | LC-MS/MS analysis of dextromethorphan metabolism in human saliva and urine to determine CYP2D6 phenotype and individual variability in N-demethylation and glucuronidation J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Dec 25;813(1-2):217-25. doi: 10.1016/j.jchromb.2004.09.040. | ||||
| 1165 | Classics in Chemical Neuroscience: Dextromethorphan (DXM). ACS Chem Neurosci. 2023 Jun 21;14(12):2256-2270. doi: 10.1021/acschemneuro.3c00088. | ||||
| 1166 | The role of CYP2D6 in primary and secondary oxidative metabolism of dextromethorphan: in vitro studies using human liver microsomes. Br J Clin Pharmacol. 1994 Sep;38(3):243-8. | ||||
| 1167 | Study on the Pharmacokinetics of Diazepam and Its Metabolites in Blood of Chinese People Eur J Drug Metab Pharmacokinet. 2020 Aug;45(4):477-485. doi: 10.1007/s13318-020-00614-8. | ||||
| 1168 | Pharmacokinetics of Diazepam and Its Metabolites in Urine of Chinese Participants. Drugs R D. 2022 Mar;22(1):43-50. doi: 10.1007/s40268-021-00375-y. | ||||
| 1169 | Kinetic disposition of diazepam and its metabolites after intravenous administration of diazepam in the horse: Relevance for doping control. J Vet Pharmacol Ther. 2021 Sep;44(5):733-744. doi: 10.1111/jvp.12991. | ||||
| 1170 | DrugBank(Pharmacology-Metabolism)Diazepam | ||||
| 1171 | Plasma and synovial fluid concentrations of diclofenac sodium and its major hydroxylated metabolites during long-term treatment of rheumatoid arthritis Eur J Clin Pharmacol. 1983;25(3):389-94. doi: 10.1007/BF01037953. | ||||
| 1172 | Simultaneous determination of diclofenac sodium and its hydroxy metabolites by capillary column gas chromatography with electron-capture detection J Chromatogr. 1981 Nov 6;217:263-71. doi: 10.1016/s0021-9673(00)88081-4. | ||||
| 1173 | DrugBank(Pharmacology-Metabolism)Diclofenac sodium | ||||
| 1174 | LABEL: NATAZIA- estradiol valerate and estradiol valerate/dienogest kit | ||||
| 1175 | Metabolism of digoxin and digoxigenin digitoxosides in rat liver microsomes: involvement of cytochrome P4503A Xenobiotica. 1999 Feb;29(2):171-85. doi: 10.1080/004982599238722. | ||||
| 1176 | Desacetyl-diltiazem displays severalfold higher affinity to CYP2D6 compared with CYP3A4. Drug Metab Dispos. 2002 Jan;30(1):1-3. | ||||
| 1177 | Pharmacokinetics and first-pass metabolism of levomepromazine in the rat Acta Pharmacol Toxicol (Copenh). 1982 Feb;50(2):148-54. doi: 10.1111/j.1600-0773.1982.tb00956.x. | ||||
| 1178 | The influence of amitriptyline and carbamazepine on levomepromazine metabolism in human liver: an in vitro study Pharmacol Rep. 2014 Dec;66(6):1122-6. doi: 10.1016/j.pharep.2014.07.012. | ||||
| 1179 | LABEL:ISOPYRAMIDE PHOSPHATE capsule, gelatin coated | ||||
| 1180 | Identification of the human P-450 enzymes responsible for the sulfoxidation and thiono-oxidation of diethyldithiocarbamate methyl ester: role of P-450 enzymes in disulfiram bioactivation. Alcohol Clin Exp Res. 1998 Sep;22(6):1212-9. | ||||
| 1181 | Increased activity of CYP3A enzyme in primary cultures of rat hepatocytes treated with docetaxel: comparative evaluation with paclitaxel Cancer Chemother Pharmacol. 2001 Aug;48(2):115-22. doi: 10.1007/s002800100283. | ||||
| 1182 | LABEL:OCETAXEL ANHYDROUS injection | ||||
| 1183 | Dofetilide, a novel class III antiarrhythmic agent J Cardiovasc Pharmacol. 1992;20 Suppl 2:S96-105. | ||||
| 1184 | Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor dolutegravir in humans | ||||
| 1185 | DrugBank(Pharmacology-Metabolism): Doravirine | ||||
| 1186 | Doxapram metabolism in human fetal hepatic organ culture | ||||
| 1187 | Identification of doxapram metabolites using high pressure ion exchange chromatography and mass spectroscopy | ||||
| 1188 | CYP2C-catalyzed delta9-tetrahydrocannabinol metabolism: kinetics, pharmacogenetics and interaction with phenytoin. Biochem Pharmacol. 2005 Oct 1;70(7):1096-103. | ||||
| 1189 | Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids | ||||
| 1190 | Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions | ||||
| 1191 | Identification of metabolic pathways and enzyme systems involved in the in vitro human hepatic metabolism of dronedarone, a potent new oral antiarrhythmic drug. Pharmacol Res Perspect. 2014 Jun;2(3):e00044. | ||||
| 1192 | DrugBank(Pharmacology-Metabolism):Dronedarone hydrochloride | ||||
| 1193 | Dydrogesterone metabolism in human liver by aldo-keto reductases and cytochrome P450 enzymes Xenobiotica. 2016 Oct;46(10):868-74. doi: 10.3109/00498254.2015.1134852. | ||||
| 1194 | DrugBank(Pharmacology-Metabolism)Estradiol acetate | ||||
| 1195 | Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005 Sep 28;227(2):115-24. | ||||
| 1196 | Involvement of CYP2J2 and CYP4F12 in the metabolism of ebastine in human intestinal microsomes | ||||
| 1197 | Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans Drug Metab Dispos. 2012 Dec;40(12):2250-5. doi: 10.1124/dmd.112.046888. | ||||
| 1198 | Biotransformation of Efavirenz and Proteomic Analysis of Cytochrome P450s and UDP-Glucuronosyltransferases in Mouse, Macaque, and Human Brain-Derived In Vitro Systems. Drug Metab Dispos. 2023 Apr;51(4):521-531. doi: 10.1124/dmd.122.001195. | ||||
| 1199 | Liver Cirrhosis Affects the Pharmacokinetics of the Six Substrates of the Basel?Phenotyping Cocktail Differently. Clin Pharmacokinet. 2022 Jul;61(7):1039-1055. doi: 10.1007/s40262-022-01119-0. | ||||
| 1200 | Instability of Efavirenz Metabolites Identified During Method Development and Validation. J Pharm Sci. 2021 Oct;110(10):3362-3366. doi: 10.1016/j.xphs.2021.06.028. | ||||
| 1201 | Eletriptan metabolism by human hepatic CYP450 enzymes and transport by human P-glycoprotein. Drug Metab Dispos. 2003 Jul;31(7):861-9. | ||||
| 1202 | #N/A | ||||
| 1203 | In vitro resistance selections using elvitegravir, raltegravir, and two metabolites of elvitegravir M1 and M4 | ||||
| 1204 | Disposition, profiling and identification of emixustat and its metabolites in humans | ||||
| 1205 | Absorption and cleavage of enalapril, a carboxyl ester prodrug, in the rat intestine: in vitro, in situ intestinal perfusion and portal vein cannulation models. Biopharm Drug Dispos. 2015 Sep;36(6):385-397. | ||||
| 1206 | LC-MS/MS reveals the formation of iminium and quinone methide reactive intermediates in entrectinib metabolism: In vivo and in vitro metabolic investigation | ||||
| 1207 | Pharmacokinetic Aspects of the Two Novel Oral Drugs Used for Metastatic Castration-Resistant Prostate Cancer: Abiraterone Acetate and Enzalutamide | ||||
| 1208 | Clinical Pharmacokinetic Studies of Enzalutamide | ||||
| 1209 | Absorption, Distribution, Metabolism, and Excretion of the Androgen Receptor Inhibitor Enzalutamide in Rats and Dogs | ||||
| 1210 | Oxidative metabolism of dehydroepiandrosterone (DHEA) and biologically active oxygenated metabolites of DHEA and epiandrosterone (EpiA)--recent reports | ||||
| 1211 | Simultaneous measurement of epinastine and its metabolite, 9,13b-dehydroepinastine, in human plasma by a newly developed ultra-performance liquid chromatography-tandem mass spectrometry and its application to pharmacokinetic studies Biomed Chromatogr. 2020 Sep;34(9):e4848. doi: 10.1002/bmc.4848. | ||||
| 1212 | The relative role of CYP3A4 and CYP3A5 in eplerenone metabolism. Toxicol Lett. 2019 Oct 15;315:9-13. | ||||
| 1213 | Ergot alkaloid mycotoxins: physiological effects, metabolism and distribution of the residual toxin in mice Sci Rep. 2020 Jun 16;10(1):9714. doi: 10.1038/s41598-020-66358-2. | ||||
| 1214 | In Vitro Characterization of Ertugliflozin Metabolism by UDP-Glucuronosyltransferase and Cytochrome P450 Enzymes Drug Metab Dispos. 2020 Dec;48(12):1350-1363. doi: 10.1124/dmd.120.000171. | ||||
| 1215 | Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF-04971729) in healthy male subjects Drug Metab Dispos. 2013 Feb;41(2):445-56. doi: 10.1124/dmd.112.049551. | ||||
| 1216 | PharmGKB summary: Macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics Pharmacogenet Genomics. 2017 Apr;27(4):164-167. doi: 10.1097/FPC.0000000000000270. | ||||
| 1217 | Effect of ABCC2 (MRP2) transport function on erythromycin metabolism Clin Pharmacol Ther. 2011 May;89(5):693-701. doi: 10.1038/clpt.2011.25. | ||||
| 1218 | Expression and characterization of human cytochrome P450 4F11: putative role in the metabolism of therapeutic drugs and eicosanoids. Toxicol Appl Pharmacol. 2004 Sep 15;199(3):295-304. | ||||
| 1219 | Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram Drug Metab Dispos. 2001 Aug;29(8):1102-9. | ||||
| 1220 | Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole Clin Pharmacokinet. 2001;40(6):411-26. doi: 10.2165/00003088-200140060-00003. | ||||
| 1221 | The metabolism of estradiol; oral compared to intravenous administration J Steroid Biochem. 1985 Dec;23(6A):1065-70. doi: 10.1016/0022-4731(85)90068-8. | ||||
| 1222 | Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003 Aug;144(8):3382-98. | ||||
| 1223 | Development of a novel tandem mass spectrometry method for the quantification of eszopiclone without interference from 2-amino-5-chloropyridine and application in a pharmacokinetic study of rat J Pharm Biomed Anal. 2020 Sep 5;188:113363. doi: 10.1016/j.jpba.2020.113363. | ||||
| 1224 | Sulfotransferase 1E1 is a low km isoform mediating the 3-O-sulfation of ethinyl estradiol Drug Metab Dispos. 2004 Nov;32(11):1299-303. doi: 10.1124/dmd.32.11.. | ||||
| 1225 | Genetic variation in the first-pass metabolism of ethinylestradiol, sex hormone binding globulin levels and venous thrombosis risk Eur J Intern Med. 2017 Jul;42:54-60. doi: 10.1016/j.ejim.2017.05.019. | ||||
| 1226 | Metabolism of 17 alpha-ethinylestradiol by human liver microsomes in vitro: aromatic hydroxylation and irreversible protein binding of metabolites J Clin Endocrinol Metab. 1974 Dec;39(6):1072-80. doi: 10.1210/jcem-39-6-1072. | ||||
| 1227 | Chiral aspects of the human metabolism of ethosuximide Biopharm Drug Dispos. 2005 Sep;26(6):225-32. doi: 10.1002/bdd.454. | ||||
| 1228 | The metabolism of ethosuximide Eur J Drug Metab Pharmacokinet. 1993 Oct-Dec;18(4):349-53. doi: 10.1007/BF03190184. | ||||
| 1229 | Reactive metabolites of the anticonvulsant drugs and approaches to minimize the adverse drug reaction. Eur J Med Chem. 2021 Dec 15;226:113890. doi: 10.1016/j.ejmech.2021.113890. | ||||
| 1230 | Integrated population pharmacokinetics of etirinotecan pegol and its four metabolites in cancer patients with solid tumors | ||||
| 1231 | Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J Clin Pharm Ther. 2007 Aug;32(4):333-41. | ||||
| 1232 | Evaluation of the absorption, excretion and metabolism of [14C] etoperidone in man | ||||
| 1233 | Reactions of glutathione with the catechol, the ortho-quinone and the semi-quinone free radical of etoposide. Consequences for DNA inactivation. Biochem Pharmacol. 1992 Apr 15;43(8):1761-8. | ||||
| 1234 | UDP-glucuronosyltransferase 1A1 is the principal enzyme responsible for etoposide glucuronidation in human liver and intestinal microsomes: structural characterization of phenolic and alcoholic glucuronides of etoposide and estimation of enzyme kinetics. Drug Metab Dispos. 2007 Mar;35(3):371-80. | ||||
| 1235 | Absorption, metabolism, and excretion of etoricoxib, a potent and selective cyclooxygenase-2 inhibitor, in healthy male volunteers | ||||
| 1236 | Biotransformation of the antiretroviral drug etravirine: metabolite identification, reaction phenotyping, and characterization of autoinduction of cytochrome P450-dependent metabolism. Drug Metab Dispos. 2012 Apr;40(4):803-14. | ||||
| 1237 | Clinical pharmacokinetics and pharmacodynamics of etravirine. Clin Pharmacokinet. 2009;48(9):561-74. | ||||
| 1238 | Identification of everolimus metabolite patterns in trough blood samples of kidney transplant patients Ther Drug Monit. 2007 Oct;29(5):592-9. doi: 10.1097/FTD.0b013e3181570830. | ||||
| 1239 | Long-term cross-validation of everolimus therapeutic drug monitoring assays: the Zortracker study Ther Drug Monit. 2015 Jun;37(3):296-303. doi: 10.1097/FTD.0000000000000191. | ||||
| 1240 | LABEL:EXEMESTANE tablet | ||||
| 1241 | Identification of CYP3A4 as the predominant isoform responsible for the metabolism of ambroxol in human liver microsomes. Xenobiotica. 2000 Jan;30(1):71-80. | ||||
| 1242 | Absorption and disposition including enterohepatic circulation of (14C) roquinimex after oral administration to healthy volunteers | ||||
| 1243 | LABEL:INREBIC- fedratinib hydrochloride capsule | ||||
| 1244 | Effect of fluconazole on the pharmacokinetics of a single dose of fedratinib in healthy adults. Cancer Chemother Pharmacol. 2022 Oct;90(4):325-334. doi: 10.1007/s00280-022-04464-w. | ||||
| 1245 | A Physiologically Based Pharmacokinetic and Pharmacodynamic Model of the CYP3A4 Substrate Felodipine for Drug-Drug Interaction Modeling Pharmaceutics. 2022 Jul 15;14(7):1474. doi: 10.3390/pharmaceutics14071474. | ||||
| 1246 | U. S. FDA Label -Fenfluramine | ||||
| 1247 | Pharmacogenomics as molecular autopsy for forensic toxicology: genotyping cytochrome P450 3A4*1B and 3A5*3 for 25 fentanyl cases. J Anal Toxicol. 2005 Oct;29(7):590-8. | ||||
| 1248 | Fentanyl Absorption, Distribution, Metabolism, and Excretion: Narrative Review and Clinical Significance Related to Illicitly Manufactured Fentanyl. J Addict Med. 2023 May 17. doi: 10.1097/ADM.0000000000001185. | ||||
| 1249 | Predictors of the Rate of Illicit Fentanyl Metabolism in a Cohort of Pregnant Individuals. J Addict Med. 2023 Jan-Feb 01;17(1):85-88. doi: 10.1097/ADM.0000000000001043. | ||||
| 1250 | Bupivacaine Metabolite Can Interfere with Norfentanyl Measurement by LC-MS/MS. J Appl Lab Med. 2022 Jun 30;7(4):854-862. doi: 10.1093/jalm/jfac009. | ||||
| 1251 | Physiologically Based Pharmacokinetic Modeling Suggests Limited Drug-Drug Interaction for Fesoterodine When Coadministered With Mirabegron J Clin Pharmacol. 2019 Nov;59(11):1505-1518. doi: 10.1002/jcph.1438. | ||||
| 1252 | LABEL:FESOTERODINE FUMARATE tablet, extended release | ||||
| 1253 | Finasteride for hair loss: a review J Dermatolog Treat. 2022 Jun;33(4):1938-1946. doi: 10.1080/09546634.2021.1959506. | ||||
| 1254 | LABEL:FINASTERIDE tablet | ||||
| 1255 | DrugBank(Pharmacology-Metabolism):Finerenone | ||||
| 1256 | Flibanserin therapy and CYP2C19 genotype. | ||||
| 1257 | LABEL:DDYI- flibanserin tablet, film coated | ||||
| 1258 | Flunitrazepam metabolism by cytochrome P450S 2C19 and 3A4. Drug Metab Dispos. 2001 Apr;29(4 Pt 1):460-5. | ||||
| 1259 | Flunitrazepam oxidative metabolism in human liver microsomes: involvement of CYP2C19 and CYP3A4. Xenobiotica. 1999 Oct;29(10):973-86. | ||||
| 1260 | Lack of interaction of buprenorphine with flunitrazepam metabolism | ||||
| 1261 | DrugBank(Pharmacology-Metabolism):Fluoxetine hydrochloride | ||||
| 1262 | (R)-, (S)-, and racemic fluoxetine N-demethylation by human cytochrome P450 enzymes. Drug Metab Dispos. 2000 Oct;28(10):1187-91. | ||||
| 1263 | Simultaneous identification and quantitation of fluoxetine and its metabolite, norfluoxetine, in biological samples by GC-MS | ||||
| 1264 | U. S. FDA Label -Fluprednisolone | ||||
| 1265 | Transport, metabolism, and hepatotoxicity of flutamide, drug-drug interaction with acetaminophen involving phase I and phase II metabolites | ||||
| 1266 | DrugBank(Pharmacology-Metabolism):Fluticasone propionate | ||||
| 1267 | Roles of different CYP enzymes in the formation of specific fluvastatin metabolites by human liver microsomes. Basic Clin Pharmacol Toxicol. 2009 Nov;105(5):327-32. | ||||
| 1268 | Metabolism and transport of oxazaphosphorines and the clinical implications Drug Metab Rev. 2005;37(4):611-703. doi: 10.1080/03602530500364023. | ||||
| 1269 | Assessment of ifosfamide pharmacokinetics, toxicity, and relation to CYP3A4 activity as measured by the erythromycin breath test in patients with sarcoma Cancer. 2007 Jun 1;109(11):2315-22. doi: 10.1002/cncr.22669. | ||||
| 1270 | DrugBank(Pharmacology-Metabolism)Fostamatinib | ||||
| 1271 | Esophageal bezoar due to karaya gum granules used as a laxative | ||||
| 1272 | Incarceration of the medial collateral ligament in the intercondylar notch following proximal avulsion | ||||
| 1273 | A simple and rapid infrared-assisted self enzymolysis extraction method for total flavonoid aglycones extraction from Scutellariae Radix and mechanism exploration | ||||
| 1274 | Management of postoperative junctional ectopic tachycardia in pediatric patients: a survey of 30 centers in Germany, Austria, and Switzerland | ||||
| 1275 | The metabolism and excretion of galantamine in rats, dogs, and humans Drug Metab Dispos. 2002 May;30(5):553-63. doi: 10.1124/dmd.30.5.553. | ||||
| 1276 | Purification and 1H NMR spectroscopic characterization of phase II metabolites of tolfenamic acid | ||||
| 1277 | Reactive metabolite of gefitinib activates inflammasomes: implications for gefitinib-induced idiosyncratic reaction J Toxicol Sci. 2020;45(11):673-680. doi: 10.2131/jts.45.673. | ||||
| 1278 | Minimal contribution of desmethyl-gefitinib, the major human plasma metabolite of gefitinib, to epidermal growth factor receptor (EGFR)-mediated tumour growth inhibition Xenobiotica. 2006 Jan;36(1):29-39. doi: 10.1080/00498250500523253. | ||||
| 1279 | An investigation into possible interactions among four vascular epidermal growth factor receptor-tyrosine kinase inhibitors with gefitinib. Cancer Chemother Pharmacol. 2021 Jan;87(1):43-52. doi: 10.1007/s00280-020-04191-0. | ||||
| 1280 | The metabolism of gemfibrozil Proc R Soc Med. 1976;69 Suppl 2(Suppl 2):11-4. | ||||
| 1281 | Intracellular pharmacokinetics of gemcitabine, its deaminated metabolite 2',2'-difluorodeoxyuridine and their nucleotides Br J Clin Pharmacol. 2018 Jun;84(6):1279-1289. doi: 10.1111/bcp.13557. | ||||
| 1282 | Biodegradation of emerging organic pollutant gemfibrozil: Mechanism, kinetics and pathway modelling. Bioresour Technol. 2023 Apr;374:128749. doi: 10.1016/j.biortech.2023.128749. | ||||
| 1283 | Metabolic Activation of Gemfibrozil Mediated by Cytochrome P450 Enzymes and Sulfotransferases. Chem Res Toxicol. 2022 Jul 18;35(7):1257-1266. doi: 10.1021/acs.chemrestox.2c00054. | ||||
| 1284 | Kinetics of glyburide metabolism by hepatic and placental microsomes of human and baboon | ||||
| 1285 | Identification of CYP3A7 for glyburide metabolism in human fetal livers. Biochem Pharmacol. 2014 Dec 15;92(4):690-700. | ||||
| 1286 | Identification of glyburide metabolites formed by hepatic and placental microsomes of humans and baboons | ||||
| 1287 | Contribution of cytochrome P450 isoforms to gliquidone metabolism in rats and human | ||||
| 1288 | Characterisation of the cytochrome P450 enzymes involved in the in vitro metabolism of granisetron. Br J Clin Pharmacol. 1994 Dec;38(6):557-66. | ||||
| 1289 | Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy | ||||
| 1290 | The metabolism and disposition of GSK2140944 in healthy human subjects | ||||
| 1291 | Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet. 2009;48(12):761-804. | ||||
| 1292 | Metabolite profiling of guanfacine in plasma and urine of healthy Japanese subjects after oral administration of guanfacine extended-release tablets | ||||
| 1293 | Determination of 3-hydroxy-guanfacine in biological fluids by electron-capture gas-liquid chromatography | ||||
| 1294 | Pharmacokinetics and metabolism of guanfacine in man: a review | ||||
| 1295 | The effects of ritonavir and lopinavir/ritonavir on the pharmacokinetics of a novel CCR5 antagonist, aplaviroc, in healthy subjects | ||||
| 1296 | NCI Thesaurus (NCIt): Halcinonide | ||||
| 1297 | Halofantrine metabolism in microsomes in man: major role of CYP 3A4 and CYP 3A5. J Pharm Pharmacol. 1999 Apr;51(4):419-26. | ||||
| 1298 | DrugBank(Pharmacology-Metabolism):Haloperidol decanoate | ||||
| 1299 | Update on hydrocodone metabolites in rats and dogs aided with a semi-automatic software for metabolite identification Mass-MetaSite | ||||
| 1300 | Role of individual human cytochrome P450 enzymes in the in vitro metabolism of hydromorphone. Xenobiotica. 2004 Apr;34(4):335-44.; | ||||
| 1301 | The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects | ||||
| 1302 | Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob Agents Chemother. 2009 Apr;53(4):1468-75. | ||||
| 1303 | Understanding the pharmacokinetics of anxiolytic drugs | ||||
| 1304 | The Role of Alcohol Dehydrogenase in Drug Metabolism: Beyond Ethanol Oxidation | ||||
| 1305 | Pharmacokinetics and metabolism of NO-1886, a lipoprotein lipase-promoting agent, in cynomolgus monkey | ||||
| 1306 | Identification of Novel Pathways in Idelalisib Metabolism and Bioactivation | ||||
| 1307 | Clinical drug interaction profile of idelalisib in healthy subjects | ||||
| 1308 | 2-Chloroacetaldehyde-induced cerebral glutathione depletion and neurotoxicity | ||||
| 1309 | Iloperidone: chemistry, pharmacodynamics, pharmacokinetics and metabolism, clinical efficacy, safety and tolerability, regulatory affairs, and an opinion. Expert Opin Drug Metab Toxicol. 2010 Dec;6(12):1551-64. | ||||
| 1310 | Role of cytochrome P450 activity in the fate of anticancer agents and in drug resistance: focus on tamoxifen, paclitaxel and imatinib metabolism | ||||
| 1311 | Participation of CYP2C8 and CYP3A4 in the N-demethylation of imatinib in human hepatic microsomes | ||||
| 1312 | In vitro biotransformation of imatinib by the tumor expressed CYP1A1 and CYP1B1 | ||||
| 1313 | Eight inhibitory monoclonal antibodies define the role of individual P-450s in human liver microsomal diazepam, 7-ethoxycoumarin, and imipramine metabolism. Drug Metab Dispos. 1999 Jan;27(1):102-9. | ||||
| 1314 | Imipramine metabolites in blood of patients during therapy and after overdose | ||||
| 1315 | Deletion of the mouse Fmo1 gene results in enhanced pharmacological behavioural responses to imipramine | ||||
| 1316 | Comparative Pharmacokinetics and Safety of Imrecoxib, a Novel Selective Cyclooxygenase-2 Inhibitor, in Elderly Healthy Subjects. Drug Des Devel Ther. 2022 Nov 8;16:3865-3876. doi: 10.2147/DDDT.S387508. | ||||
| 1317 | Roles of UGT, P450, and Gut Microbiota in the Metabolism of Epacadostat in Humans | ||||
| 1318 | Vasorelaxant actions of 5-OH-indapamide, a major metabolite of indapamide: comparison with indapamide, hydrochlorothiazide and cicletanine Eur J Pharmacol. 1994 Apr 21;256(2):185-91. doi: 10.1016/0014-2999(94)90244-5. | ||||
| 1319 | Hepatic and intestinal metabolism of indinavir, an HIV protease inhibitor, in rat and human microsomes. Major role of CYP3A. Biochem Pharmacol. 1997 Apr 25;53(8):1187-95. | ||||
| 1320 | Involvement of CYP3A4 and MDR1 in altered metabolism and transport of indinavir in 1,25(OH)(2)D(3)-treated Caco-2 cells. Eur J Pharm Sci. 2023 Apr 1;183:106396. doi: 10.1016/j.ejps.2023.106396. | ||||
| 1321 | In vitro metabolism of indiplon and an assessment of its drug interaction potential | ||||
| 1322 | VMAT2 Inhibitors in Neuropsychiatric Disorders | ||||
| 1323 | Biotransformation of the ipecac alkaloids cephaeline and emetine from ipecac syrup in rats Eur J Drug Metab Pharmacokinet. 2002 Jan-Mar;27(1):29-35. doi: 10.1007/BF03190402. | ||||
| 1324 | Pharmacokinetics, metabolism, and excretion of irinotecan (CPT-11) following I.V. infusion of [(14)C]CPT-11 in cancer patients Drug Metab Dispos. 2000 Apr;28(4):423-33. | ||||
| 1325 | Effect of UGT1A1, CYP3A and CES Activities on the Pharmacokinetics of Irinotecan and its Metabolites in Patients with UGT1A1 Gene Polymorphisms. Eur J Drug Metab Pharmacokinet. 2021 Mar;46(2):317-324. doi: 10.1007/s13318-021-00675-3. | ||||
| 1326 | Structural insights into catalytical capability for CPT11 hydrolysis and substrate specificity of a novel marine microbial carboxylesterase, E93. Front Microbiol. 2023 Jan 11;13:1081094. doi: 10.3389/fmicb.2022.1081094. | ||||
| 1327 | Population pharmacokinetic model of irinotecan and its four main metabolites in patients treated with FOLFIRI or FOLFIRINOX regimen. Cancer Chemother Pharmacol. 2021 Aug;88(2):247-258. doi: 10.1007/s00280-021-04255-9. | ||||
| 1328 | Concomitant use of isavuconazole and CYP3A4/5 inducers: Where pharmacogenetics meets pharmacokinetics Mycoses. 2021 Sep;64(9):1111-1116. doi: 10.1111/myc.13300. | ||||
| 1329 | Pharmacokinetics of isosorbide mononitrate Am J Cardiol. 1992 Nov 27;70(17):61G-66G. doi: 10.1016/0002-9149(92)90028-w. | ||||
| 1330 | Human biliary metabolites of isotretinoin: identification, quantification, synthesis, and biological activity Xenobiotica. 1990 Feb;20(2):193-207. doi: 10.3109/00498259009047155. | ||||
| 1331 | Isotretinoin and its Metabolites Alter mRNA of Multiple Enzyme and Transporter Genes In Vitro, but Downregulation of Organic Anion Transporting Polypeptide Does Not Translate to the Clinic Drug Metab Dispos. 2022 Jul;50(7):1042-1052. doi: 10.1124/dmd.122.000882. | ||||
| 1332 | The use of isotretinoin in acne Dermatoendocrinol. 2009 May;1(3):162-9. doi: 10.4161/derm.1.3.9364. | ||||
| 1333 | The effect of CYP3A4 genetic polymorphism and drug interaction on the metabolism of istradefylline Chem Biol Interact. 2022 Oct 1;366:110123. doi: 10.1016/j.cbi.2022.110123. | ||||
| 1334 | An evaluation of lumateperone tosylate for the treatment of schizophrenia | ||||
| 1335 | Role of itraconazole metabolites in CYP3A4 inhibition. Drug Metab Dispos. 2004 Oct;32(10):1121-31. | ||||
| 1336 | Accurate prediction of dose-dependent CYP3A4 inhibition by itraconazole and its metabolites from in vitro inhibition data Clin Pharmacol Ther. 2010 Oct;88(4):499-505. doi: 10.1038/clpt.2010.119. | ||||
| 1337 | Repurposing antifungals: population pharmacokinetics of itraconazole and hydroxy-itraconazole following administration of a nanocrystal formulation. J Antimicrob Chemother. 2023 May 3;78(5):1219-1224. doi: 10.1093/jac/dkad072. | ||||
| 1338 | Does metabolite matter? Defining target itraconazole and hydroxy-itraconazole serum concentrations for blastomycosis. Mycoses. 2023 May;66(5):412-419. doi: 10.1111/myc.13565. | ||||
| 1339 | Influence of Gender, Body Mass Index, and Age on the Pharmacokinetics of Itraconazole in Healthy Subjects: Non-Compartmental Versus Compartmental Analysis. Front Pharmacol. 2022 Jun 15;13:796336. doi: 10.3389/fphar.2022.796336. | ||||
| 1340 | Simultaneous determination of ivabradine and its metabolites in human plasma by liquid chromatography--tandem mass spectrometry J Chromatogr B Biomed Sci Appl. 2000 Aug 18;745(2):261-9. doi: 10.1016/s0378-4347(00)00275-9. | ||||
| 1341 | PharmGKB summary: ivacaftor pathway, pharmacokinetics/pharmacodynamics Pharmacogenet Genomics. 2017 Jan;27(1):39-42. doi: 10.1097/FPC.0000000000000246. | ||||
| 1342 | Identification of the metabolites of ivermectin in humans Pharmacol Res Perspect. 2021 Feb;9(1):e00712. doi: 10.1002/prp2.712. | ||||
| 1343 | PubChem : JNJ-54135419 | ||||
| 1344 | [Metabolism of lomerizine hydrochloride (KB-2796) in rats] | ||||
| 1345 | The clinical toxicology of ketamine. Clin Toxicol (Phila). 2023 Jun;61(6):415-428. doi: 10.1080/15563650.2023.2212125. | ||||
| 1346 | Pharmacokinetics of Ketamine Transfer Into Human Milk. J Clin Psychopharmacol. 2023 May 23. doi: 10.1097/JCP.0000000000001711. | ||||
| 1347 | Chronic exposure to (2?R,6?R)-hydroxynorketamine induces developmental neurotoxicity in hESC-derived cerebral organoids. J Hazard Mater. 2023 Jul 5;453:131379. doi: 10.1016/j.jhazmat.2023.131379. | ||||
| 1348 | Urinary diazepam metabolite distribution in a chronic pain population J Anal Toxicol. 2014 Apr;38(3):135-42. doi: 10.1093/jat/bku001. | ||||
| 1349 | Revisiting the metabolism of ketoconazole using accurate mass Drug Metab Lett. 2009 Aug;3(3):191-8. doi: 10.2174/187231209789352085. | ||||
| 1350 | Label:KLH-2109 | ||||
| 1351 | DrugBank : KRM-1648 | ||||
| 1352 | Identification of the cytochrome P450 enzymes involved in the metabolism of domperidone. Xenobiotica. 2004 Nov-Dec;34(11-12):1013-23. | ||||
| 1353 | Absorption, disposition, metabolic fate and elimination of the anti-epileptic drug lacosamide in humans: mass balance following intravenous and oral administration Eur J Drug Metab Pharmacokinet. 2012 Dec;37(4):241-8. doi: 10.1007/s13318-012-0093-x. | ||||
| 1354 | Identification of the human P450 enzymes involved in lansoprazole metabolism. J Pharmacol Exp Ther. 1996 May;277(2):805-16. | ||||
| 1355 | Oxidative metabolism of lansoprazole by human liver cytochromes P450. Mol Pharmacol. 1995 Feb;47(2):410-8. | ||||
| 1356 | Metabolic intermediate complex formation of human cytochrome P450 3A4 by lapatinib Drug Metab Dispos. 2011 Jun;39(6):1022-30. doi: 10.1124/dmd.110.037531. | ||||
| 1357 | Fenretinide metabolism in humans and mice: utilizing pharmacological modulation of its metabolic pathway to increase systemic exposure | ||||
| 1358 | Lefamulin: a New Hope in the Field of Community-Acquired Bacterial Pneumonia. Curr Pharmacol Rep. 2022;8(6):418-426. doi: 10.1007/s40495-022-00297-6. | ||||
| 1359 | Development, validation, and application of an LC-MS/MS method for the quantification of the novel antibiotic drug lefamulin (Xenleta?) and its main metabolite 2R-hydroxy lefamulin in human plasma. J Pharm Biomed Anal. 2021 Oct 25;205:114293. doi: 10.1016/j.jpba.2021.114293. | ||||
| 1360 | The impact of individual human cytochrome P450 enzymes on oxidative metabolism of anticancer drug lenvatinib | ||||
| 1361 | Absence of excretion of the active moiety of bisacodyl and sodium picosulfate into human breast milk: an open-label, parallel-group, multiple-dose study in healthy lactating women Drug Metab Pharmacokinet. 2011;26(5):458-64. doi: 10.2133/dmpk.dmpk-11-rg-007. | ||||
| 1362 | N-demethylation of levo-alpha-acetylmethadol by human placental aromatase. Biochem Pharmacol. 2004 Mar 1;67(5):885-92. | ||||
| 1363 | The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuropharmacol. 2016;14(2):191-9. | ||||
| 1364 | [Influence of CYP3A4/5 polymorphisms in the pharmacokinetics of levonorgestrel: a pilot study]. Biomedica. 2012 Oct-Dec;32(4):570-7. | ||||
| 1365 | The Use and Method of Action of Intravenous Lidocaine and Its Metabolite in Headache Disorders Headache. 2018 May;58(5):783-789. doi: 10.1111/head.13298. | ||||
| 1366 | Involvement of CYP1A2 and CYP3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab Dispos. 2000 Aug;28(8):959-65. | ||||
| 1367 | Identification of cytochrome P450 isoforms involved in the metabolism of loperamide in human liver microsomes. Eur J Clin Pharmacol. 2004 Oct;60(8):575-81. | ||||
| 1368 | Advances in high-resolution MS and hepatocyte models solve a long-standing metabolism challenge: the loratadine story. Bioanalysis. 2016 Aug;8(16):1645-62. | ||||
| 1369 | #N/A | ||||
| 1370 | Pharmacogenomics of statins: understanding susceptibility to adverse effects. Pharmgenomics Pers Med. 2016 Oct 3;9:97-106. | ||||
| 1371 | Identification of sulfonyl-loxoprofen as novel phase 2 conjugate in rat | ||||
| 1372 | DrugBank : LS-248 | ||||
| 1373 | Dopamine D(2) receptor occupancy of lumateperone (ITI-007): a Positron Emission Tomography Study in patients with schizophrenia | ||||
| 1374 | Design, synthesis, and biological evaluation of optimized phthalazine derivatives as hedgehog signaling pathway inhibitors | ||||
| 1375 | In vitro metabolism of perospirone in rat, monkey and human liver microsomes | ||||
| 1376 | Clinical pharmacokinetics and pharmacodynamics of the endothelin receptor antagonist macitentan | ||||
| 1377 | Preclinical pharmacokinetics and metabolism of MAK683, a clinical stage selective oral embryonic ectoderm development (EED) inhibitor for cancer treatment | ||||
| 1378 | Antimicrobial Resistance, Virulence Factor-Encoding Genes, and Biofilm-Forming Ability of Community-Associated Uropathogenic Escherichia coli in Western Saudi Arabia | ||||
| 1379 | Meals, Microbiota and Mental Health in Children and Adolescents (MMM-Study): A protocol for an observational longitudinal case-control study | ||||
| 1380 | Corrigendum to: Physiologically-based pharmacokinetic modeling to evaluate in vitro-to-in vivo extrapolation for intestinal P-glycoprotein inhibition | ||||
| 1381 | Transient and resident pathogens: Intra-facility genetic diversity of Listeria monocytogenes and Salmonella from food production environments | ||||
| 1382 | IgG and IgM Immunohistochemistry in Primary Biliary Cholangitis (PBC) and Autoimmune Hepatitis (AIH) Liver Explants | ||||
| 1383 | In vitro and in vivo pharmacokinetic characterization of mavacamten, a first-in-class small molecule allosteric modulator of beta cardiac myosin | ||||
| 1384 | A prospective longitudinal study of growth in twin gestations compared with growth in singleton pregnancies. I. The fetal head J Ultrasound Med. 1991 Aug;10(8):439-43. doi: 10.7863/jum.1991.10.8.439. | ||||
| 1385 | Metabolism of Meloxicam in human liver involves cytochromes P4502C9 and 3A4. Xenobiotica. 1998 Jan;28(1):1-13. | ||||
| 1386 | Meperidine and normeperidine levels following meperidine administration during labor. I. Mother | ||||
| 1387 | Effects of ethinyl estradiol on pharmacokinetics of meperidine and pentobarbital in the rat | ||||
| 1388 | Impact of cytochrome P450 variation on meperidine N-demethylation to the neurotoxic metabolite normeperidine. Xenobiotica. 2020 Feb;50(2):209-222. | ||||
| 1389 | Metamizole (Dipyrone) and the Liver: A Review of the Literature J Clin Pharmacol. 2019 Nov;59(11):1433-1442. doi: 10.1002/jcph.1512. | ||||
| 1390 | Clinical pharmacokinetics of dipyrone and its metabolites Clin Pharmacokinet. 1995 Mar;28(3):216-34. doi: 10.2165/00003088-199528030-00004. | ||||
| 1391 | Effects of cytochrome P450 single nucleotide polymorphisms on methadone metabolism and pharmacodynamics | ||||
| 1392 | Identification of novel metoclopramide metabolites in humans: in vitro and in vivo studies | ||||
| 1393 | The role of human cytochrome P450 enzymes in the formation of 2-hydroxymetronidazole: CYP2A6 is the high affinity (low Km) catalyst. Drug Metab Dispos. 2013 Sep;41(9):1686-94. | ||||
| 1394 | Disposition and metabolism of metronidazole in patients with liver failure | ||||
| 1395 | Metronidazole Metabolism in Neonates and the Interplay Between Ontogeny and Genetic Variation | ||||
| 1396 | Metronidazole: an update on metabolism, structure-cytotoxicity and resistance mechanisms | ||||
| 1397 | Heterotropic activation of the midazolam hydroxylase activity of CYP3A by a positive allosteric modulator of mGlu5: in vitro to in vivo translation and potential impact on clinically relevant drug-drug interactions | ||||
| 1398 | The Oral Bioavailability and Metabolism of Midazolam in Stable Critically Ill Children: A Pharmacokinetic Microtracing Study | ||||
| 1399 | Midostaurin: an emerging treatment for acute myeloid leukemia patients | ||||
| 1400 | Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth | ||||
| 1401 | Respirator performance terminology | ||||
| 1402 | PAT Program report: background and current status. Proficiency Analytical Testing | ||||
| 1403 | Studies on the cytochrome P450 (CYP)-mediated metabolic properties of miocamycin: evaluation of the possibility of a metabolic intermediate complex formation with CYP, and identification of the human CYP isoforms | ||||
| 1404 | FDA:Mirvetuximab soravtansine | ||||
| 1405 | DrugBank(Pharmacology-Metabolism):Modafinil | ||||
| 1406 | In vitro metabolism of montelukast by cytochrome P450s and UDP-glucuronosyltransferases. Drug Metab Dispos. 2015 Dec;43(12):1905-16. | ||||
| 1407 | Population pharmacokinetic modeling of motesanib and its active metabolite, M4, in cancer patients | ||||
| 1408 | Metabolism and disposition of the dipeptidyl peptidase IV inhibitor teneligliptin in humans | ||||
| 1409 | Development of a liquid chromatography/tandem-mass spectrometry assay for the simultaneous determination of teneligliptin and its active metabolite teneligliptin sulfoxide in human plasma | ||||
| 1410 | DrugBank(Pharmacology-Metabolism):Adagrasib | ||||
| 1411 | DrugBank(Pharmacology-Metabolism):Mycophenolate mofetil | ||||
| 1412 | Identification of human cytochrome P450 isozymes involved in the metabolism of naftopidil enantiomers in vitro. J Pharm Pharmacol. 2014 Nov;66(11):1534-51. | ||||
| 1413 | Characterization of Ca(2+)-antagonistic effects of three metabolites of the new antihypertensive agent naftopidil, (naphthyl)hydroxy-naftopidil, (phenyl)hydroxy-naftopidil, and O-desmethyl-naftopidil J Cardiovasc Pharmacol. 1991 Dec;18(6):918-25. doi: 10.1097/00005344-199112000-00020. | ||||
| 1414 | LABEL:Nalmefene | ||||
| 1415 | Absorption, distribution, metabolism, and excretion of [14C]-labeled naloxegol in healthy subjects | ||||
| 1416 | Pharmacokinetics of nateglinide enantiomers and their metabolites in Goto-Kakizaki rats, a model for type 2 diabetes mellitus | ||||
| 1417 | DrugBank(Pharmacology-Metabolism):Nefazodone | ||||
| 1418 | Clinical pharmacokinetics of nefazodone. Clin Pharmacokinet. 1997 Oct;33(4):260-75. | ||||
| 1419 | Conversion of the HIV protease inhibitor nelfinavir to a bioactive metabolite by human liver CYP2C19 Drug Metab Dispos. 2004 Dec;32(12):1462-7. doi: 10.1124/dmd.104.001743. | ||||
| 1420 | Nestorone? as a Novel Progestin for Nonoral Contraception: Structure-Activity Relationships and Brain Metabolism Studies | ||||
| 1421 | Absorption, disposition, metabolic fate, and elimination of the dopamine agonist rotigotine in man: administration by intravenous infusion or transdermal delivery | ||||
| 1422 | Disposition and biotransformation of the antiretroviral drug nevirapine in humans Drug Metab Dispos. 1999 Aug;27(8):895-901. | ||||
| 1423 | Differences in nevirapine biotransformation as a factor for its sex-dependent dimorphic profile of adverse drug reactions J Antimicrob Chemother. 2014 Feb;69(2):476-82. doi: 10.1093/jac/dkt359. | ||||
| 1424 | Sulfation of 12-hydroxy-nevirapine by human SULTs and the effects of genetic polymorphisms of SULT1A1 and SULT2A1. Biochem Pharmacol. 2022 Oct;204:115243. doi: 10.1016/j.bcp.2022.115243. | ||||
| 1425 | U. S. FDA Label -Nevirapine | ||||
| 1426 | CYP3A5 genotype did not impact on nifedipine disposition in healthy volunteers Pharmacogenomics J. 2004;4(1):34-9. doi: 10.1038/sj.tpj.6500218. | ||||
| 1427 | Pharmacokinetics and metabolism of nifedipine Hypertension. 1983 Jul-Aug;5(4 Pt 2):II18-24. doi: 10.1161/01.hyp.5.4_pt_2.ii18. | ||||
| 1428 | The first pass metabolism of nifedipine in man Br J Clin Pharmacol. 1984 Dec;18(6):951-4. doi: 10.1111/j.1365-2125.1984.tb02569.x. | ||||
| 1429 | Enzyme kinetics and inhibition of nimodipine metabolism in human liver microsomes. Acta Pharmacol Sin. 2000 Aug;21(8):690-4. | ||||
| 1430 | NORMAN Suspect List Exchange:Nimodipine | ||||
| 1431 | Metabolic pro?ling of tyrosine kinase inhibitor nintedanib using metabolomics J Pharm Biomed Anal. 2020 Feb 20;180:113045. doi: 10.1016/j.jpba.2019.113045. | ||||
| 1432 | Clinical pharmacokinetics of nisoldipine coat-core Clin Pharmacokinet. 1998 Sep;35(3):191-208. doi: 10.2165/00003088-199835030-00003. | ||||
| 1433 | U. S. FDA Label -Nisoldipine | ||||
| 1434 | Metabolism of norgestimate by human gastrointestinal mucosa and liver microsomes in vitro J Steroid Biochem Mol Biol. 1991 Apr;38(4):497-503. doi: 10.1016/0960-0760(91)90338-6. | ||||
| 1435 | Pharmacokinetics of nortriptyline and its 10-hydroxy metabolite in Chinese subjects of different CYP2D6 genotypes Clin Pharmacol Ther. 1998 Oct;64(4):384-90. doi: 10.1016/S0009-9236(98)90069-8. | ||||
| 1436 | Dexamethasone metabolism by human liver in vitro. Metabolite identification and inhibition of 6-hydroxylation | ||||
| 1437 | Role of cytochrome P450 isoenzymes in metabolism of O(6)-benzylguanine: implications for dacarbazine activation. Clin Cancer Res. 2001 Dec;7(12):4239-44. | ||||
| 1438 | In vitro metabolism of olaparib in liver microsomes by liquid chromatography/electrospray ionization high-resolution mass spectrometry Rapid Commun Mass Spectrom. 2020 Feb 15;34(3):e8575. doi: 10.1002/rcm.8575. | ||||
| 1439 | Identification of novel ondansetron metabolites using LC/MS(n) and NMR J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Sep 15;1095:138-141. doi: 10.1016/j.jchromb.2018.07.013. | ||||
| 1440 | Pharmacodynamic and pharmacokinetic behavior of landiolol during dobutamine challenge in healthy adults | ||||
| 1441 | In Vitro Metabolism of Oprozomib, an Oral Proteasome Inhibitor: Role of Epoxide Hydrolases and Cytochrome P450s | ||||
| 1442 | Human cytochromes mediating gepirone biotransformation at low substrate concentrations. Biopharm Drug Dispos. 2003 Mar;24(2):87-94. | ||||
| 1443 | Metabolic Disposition of Osimertinib in Rats, Dogs, and Humans: Insights into a Drug Designed to Bind Covalently to a Cysteine Residue of Epidermal Growth Factor Receptor Drug Metab Dispos. 2016 Aug;44(8):1201-12. doi: 10.1124/dmd.115.069203. | ||||
| 1444 | Population Pharmacokinetics, Pharmacogenomics, and Adverse Events of Osimertinib and its Two Active Metabolites, AZ5104 and AZ7550, in Japanese Patients with Advanced Non-small Cell Lung Cancer: a Prospective Observational Study. Invest New Drugs. 2023 Feb;41(1):122-133. doi: 10.1007/s10637-023-01328-9. | ||||
| 1445 | Instability Mechanism of Osimertinib in Plasma and a Solving Strategy in the Pharmacokinetics Study. Front Pharmacol. 2022 Jul 22;13:928983. doi: 10.3389/fphar.2022.928983. | ||||
| 1446 | Cytochrome P450 enzyme activity and protein expression in primary porcine enterocyte and hepatocyte cultures. Xenobiotica. 2000 Jan;30(1):27-46. | ||||
| 1447 | Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability Drugs Aging. 1995 Mar;6(3):243-62. doi: 10.2165/00002512-199506030-00007. | ||||
| 1448 | Stereoselective pharmacokinetics of oxybutynin and N-desethyloxybutynin in vitro and in vivo. Xenobiotica. 2007 Jan;37(1):59-73. | ||||
| 1449 | Metabolism and Disposition of Prescription Opioids: A Review | ||||
| 1450 | Enzalutamide Reduces Oxycodone Exposure in Men with Prostate Cancer. Clin Pharmacokinet. 2023 May 10. doi: 10.1007/s40262-023-01255-1. | ||||
| 1451 | Impact of genetic variation in CYP2C19, CYP2D6, and CYP3A4 on oxycodone and its metabolites in a large database of clinical urine drug tests. Pharmacogenomics J. 2022 Feb;22(1):25-32. doi: 10.1038/s41397-021-00253-5. | ||||
| 1452 | DrugBank(Pharmacology-Metabolism)Oxycodone hydrochloride | ||||
| 1453 | [International council of nurses: the international voice of the nursing profession] Bol Oficina Sanit Panam. 1979 Aug;87(2):152-5. | ||||
| 1454 | Update: influenza activity--United States MMWR Morb Mortal Wkly Rep. 1986 Mar 14;35(10):166-7. | ||||
| 1455 | In vitro and in vivo metabolic investigation of the Palbociclib by UHPLC-Q-TOF/MS/MS and in silico toxicity studies of its metabolites J Pharm Biomed Anal. 2018 Aug 5;157:59-74. doi: 10.1016/j.jpba.2018.05.008. | ||||
| 1456 | Different enantioselective 9-hydroxylation of risperidone by the two human CYP2D6 and CYP3A4 enzymes. Drug Metab Dispos. 2001 Oct;29(10):1263-8. | ||||
| 1457 | S73 | METXBIODB | metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560 | ||||
| 1458 | Metabolism of systematically given corticosteroids Dermatologica. 1975;151(3):129-34. doi: 10.1159/000251325. | ||||
| 1459 | Disposition of a specific cyclooxygenase-2 inhibitor, valdecoxib, in human Drug Metab Dispos. 2002 Sep;30(9):1013-21. doi: 10.1124/dmd.30.9.1013. | ||||
| 1460 | Identification of cytochrome P450 isoforms involved in the metabolism of paroxetine and estimation of their importance for human paroxetine metabolism using a population-based simulator. Drug Metab Dispos. 2010 Mar;38(3):376-85. | ||||
| 1461 | Pazopanib: the newest tyrosine kinase inhibitor for the treatment of advanced or metastatic renal cell carcinoma. Drugs. 2011 Mar 5;71(4):443-54. | ||||
| 1462 | Bioavailability, metabolism and disposition of oral pazopanib in patients with advanced cancer Xenobiotica. 2013 May;43(5):443-53. doi: 10.3109/00498254.2012.734642. | ||||
| 1463 | Direct and Sequential Bioactivation of Pemigatinib to Reactive Iminium Ion Intermediates Culminates in Mechanism-Based Inactivation of Cytochrome P450 3A | ||||
| 1464 | Anti-Parkinson's disease drugs and pharmacogenetic considerations Expert Opin Drug Metab Toxicol. 2013 Jul;9(7):859-74. doi: 10.1517/17425255.2013.789018. | ||||
| 1465 | Pharmacokinetics, Metabolite Measurement, and Biomarker Identification of Dermal Exposure to Permethrin Using Accelerator Mass Spectrometry. Toxicol Sci. 2021 Aug 30;183(1):49-59. doi: 10.1093/toxsci/kfab082. | ||||
| 1466 | Validation of enantioseparation and quantitation of an active metabolite of abrocitinib in human plasma | ||||
| 1467 | DrugBank(Pharmacology-Metabolism)Phenacetin | ||||
| 1468 | Insights into the mechanisms of action of the MAO inhibitors phenelzine and tranylcypromine: a review J Psychiatry Neurosci. 1992 Nov;17(5):206-14. | ||||
| 1469 | DrugBank(Pharmacology-Metabolism)Phenobarbital | ||||
| 1470 | Determination of phentermine, N-hydroxyphentermine and mephentermine in urine using dilute and shoot liquid chromatography-tandem mass spectrometry J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Sep 1;1029-1030:22-27. doi: 10.1016/j.jchromb.2016.06.045. | ||||
| 1471 | The Respective Roles of CYP3A4 and CYP2D6 in the Metabolism of Pimozide to Established and Novel Metabolites Drug Metab Dispos. 2020 Nov;48(11):1113-1120. doi: 10.1124/dmd.120.000188. | ||||
| 1472 | LABEL:IMOZIDE tablet | ||||
| 1473 | Simultaneous quantitation of pioglitazone and its metabolites in human serum by liquid chromatography and solid phase extraction J Pharm Biomed Anal. 1996 Feb;14(4):465-73. doi: 10.1016/0731-7085(95)01665-1. | ||||
| 1474 | Metabolic fate of pitavastatin, a new inhibitor of HMG-CoA reductase: similarities and difference in the metabolism of pitavastatin in monkeys and humans | ||||
| 1475 | Metabolic fate of pitavastatin, a new inhibitor of HMG-CoA reductase: human UDP-glucuronosyltransferase enzymes involved in lactonization. Xenobiotica. 2003 Jan;33(1):27-41. | ||||
| 1476 | FDA Center for Drug Evaluation and Research: Pitolisant Non-clinical Review(s) | ||||
| 1477 | Wakix, INN-Pitolisant - European Medicines Agency - Europa EU | ||||
| 1478 | Absorption, metabolism and excretion of [14C]pomalidomide in humans following oral administration Cancer Chemother Pharmacol. 2013 Feb;71(2):489-501. doi: 10.1007/s00280-012-2040-6. | ||||
| 1479 | Metabolic profiles of pomalidomide in human plasma simulated with pharmacokinetic data in control and humanized-liver mice Xenobiotica. 2017 Oct;47(10):844-848. doi: 10.1080/00498254.2016.1247218. | ||||
| 1480 | Novel pathways of ponatinib disposition catalyzed by CYP1A1 involving generation of potentially toxic metabolites. J Pharmacol Exp Ther. 2017 Oct;363(1):12-19. | ||||
| 1481 | Absorption, metabolism, and excretion of [(14)C]ponatinib after a single oral dose in humans Cancer Chemother Pharmacol. 2017 Mar;79(3):507-518. doi: 10.1007/s00280-017-3240-x. | ||||
| 1482 | In vitro metabolites characterization of ponatinib in human liver microsomes using ultra-high performance liquid chromatography combined with Q-Exactive-Orbitrap tandem mass spectrometry Biomed Chromatogr. 2020 Jun;34(6):e4819. doi: 10.1002/bmc.4819. | ||||
| 1483 | In vitro metabolism and inhibitory effects of pranlukast in human liver microsomes | ||||
| 1484 | Determination of the active and inactive metabolites of prasugrel in human plasma by liquid chromatography/tandem mass spectrometry Rapid Commun Mass Spectrom. 2007;21(2):169-79. doi: 10.1002/rcm.2813. | ||||
| 1485 | Interactions of fentanyl with blood platelets and plasma proteins: platelet sensitivity to prasugrel metabolite is not affected by fentanyl under in vitro conditions. Pharmacol Rep. 2023 Apr;75(2):423-441. doi: 10.1007/s43440-023-00447-7. | ||||
| 1486 | Pharmacokinetic and Pharmacodynamic Modeling and Simulation Analysis of Prasugrel in Healthy Male Volunteers. Clin Pharmacol Drug Dev. 2023 Jan;12(1):21-29. doi: 10.1002/cpdd.1172. | ||||
| 1487 | Prazepam metabolism by man Clin Pharmacol Ther. 1970 Nov-Dec;11(6):890-7. doi: 10.1002/cpt1970116890. | ||||
| 1488 | Prazepam metabolites in dog urine J Pharm Sci. 1970 Mar;59(3):322-5. doi: 10.1002/jps.2600590309. | ||||
| 1489 | In vitro and in vivo human metabolism and pharmacokinetics of S- and R-praziquantel Pharmacol Res Perspect. 2020 Aug;8(4):e00618. doi: 10.1002/prp2.618. | ||||
| 1490 | Detection and characterization of prednisolone metabolites in human urine by LC-MS/MS J Mass Spectrom. 2015 Mar;50(3):633-42. doi: 10.1002/jms.3571. | ||||
| 1491 | FDA:Pretomanid | ||||
| 1492 | Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys. 1997 Oct 1;346(1):161-9. | ||||
| 1493 | Progesterone Metabolites Produced by Cytochrome P450 3A Modulate Uterine Contractility in a Murine Model Reprod Sci. 2015 Dec;22(12):1577-86. doi: 10.1177/1933719115589414. | ||||
| 1494 | Sensitive method for the quantitative determination of proguanil and its metabolites in rat blood and plasma by liquid chromatography-mass spectrometry | ||||
| 1495 | Single dose pharmacokinetics of proguanil and its metabolites in pregnancy | ||||
| 1496 | The muscarinic receptor antagonist propiverine exhibits (1)-adrenoceptor antagonism in human prostate and porcine trigonum | ||||
| 1497 | FDA label:Belzutifan | ||||
| 1498 | Poncirin and its metabolite ponciretin attenuate colitis in mice by inhibiting LPS binding on TLR4 of macrophages and correcting Th17/Treg imbalance | ||||
| 1499 | In vitro metabolism of quazepam in human liver and intestine and assessment of drug interactions. Xenobiotica. 2004 Nov-Dec;34(11-12):1001-11. | ||||
| 1500 | Metabolism of quetiapine by CYP3A4 and CYP3A5 in presence or absence of cytochrome B5. Drug Metab Dispos. 2009 Feb;37(2):254-8. | ||||
| 1501 | CYP450 phenotyping and metabolite identification of quinine by accurate mass UPLC-MS analysis: a possible metabolic link to blackwater fever | ||||
| 1502 | Metabolism of quinine in man: identification of a major metabolite, and effects of smoking and rifampicin pretreatment | ||||
| 1503 | Effects of Plasmodium falciparum infection on the pharmacokinetics of quinine and its metabolites in pregnant and non-pregnant Sudanese women | ||||
| 1504 | Rapid quantification of quinine and its major metabolite (3S)-3-hydroxyquinine in diluted urine by UPLC-MS/MS | ||||
| 1505 | Netupitant PET imaging and ADME studies in humans | ||||
| 1506 | Determination of rabeprazole enantiomers and their metabolites by high-performance liquid chromatography with solid-phase extraction | ||||
| 1507 | Disposition and metabolism of ralfinamide, a novel Na-channel blocker, in healthy male volunteers | ||||
| 1508 | Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions | ||||
| 1509 | Fatal, Intentional Overdose of Ranolazine: Post-Mortem Distribution of Parent Drug and its Major Metabolite | ||||
| 1510 | Comparison of pharmacokinetic parameters of ranolazine between diabetic and non-diabetic rats | ||||
| 1511 | Comparison of the disposition and of the metabolic pattern of Reboxetine, a new antidepressant, in the rat, dog, monkey and man | ||||
| 1512 | Mass balance, metabolic disposition, and pharmacokinetics of a single oral dose of regorafenib in healthy human subjects | ||||
| 1513 | Quantification of cobimetinib, cabozantinib, dabrafenib, niraparib, olaparib, vemurafenib, regorafenib and its metabolite regorafenib M2 in human plasma by UPLC-MS/MS | ||||
| 1514 | Different effects of SLCO1B1 polymorphism on the pharmacokinetics and pharmacodynamics of repaglinide and nateglinide | ||||
| 1515 | Bioanalytical method for in vitro metabolism study of repaglinide using 96-blade thin-film solid-phase microextraction and LC-MS/MS | ||||
| 1516 | Repaglinide-gemfibrozil drug interaction: inhibition of repaglinide glucuronidation as a potential additional contributing mechanism. Br J Clin Pharmacol. 2010 Dec;70(6):870-80. | ||||
| 1517 | Human biotransformation of the nonnucleoside reverse transcriptase inhibitor rilpivirine and a cross-species metabolism comparison. Antimicrob Agents Chemother. 2013 Oct;57(10):5067-79. | ||||
| 1518 | Impact of low-dose ritonavir on danoprevir pharmacokinetics: results of computer-based simulations and a clinical drug-drug interaction study | ||||
| 1519 | A Single-center, Open-label, Parallel Control Study Comparing the Pharmacokinetics and Safety of a Single Oral Dose of Roflumilast and Its Active Metabolite Roflumilast N-oxide in Healthy Chinese and Caucasian Volunteers | ||||
| 1520 | Absorption, metabolism, and excretion of the antiemetic rolapitant, a selective neurokinin-1 receptor antagonist, in healthy male subjects | ||||
| 1521 | Identification of cytochrome P450 enzymes involved in the metabolism of FK228, a potent histone deacetylase inhibitor, in human liver microsomes. Biol Pharm Bull. 2005 Jan;28(1):124-9. | ||||
| 1522 | Use of the steroid derivative RPR 106541 in combination with site-directed mutagenesis for enhanced cytochrome P-450 3A4 structure/function analysis | ||||
| 1523 | Pharmacokinetics and safety of rucaparib in patients with advanced solid tumors and hepatic impairment. Cancer Chemother Pharmacol. 2021 Aug;88(2):259-270. doi: 10.1007/s00280-021-04278-2. | ||||
| 1524 | CYP3A5*3 and MDR1 C3435T are influencing factors of inter-subject variability in rupatadine pharmacokinetics in healthy Chinese volunteers. Eur J Drug Metab Pharmacokinet. 2016 Apr;41(2):117-24. | ||||
| 1525 | Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans Drug Metab Dispos. 2010 Nov;38(11):2023-31. doi: 10.1124/dmd.110.033787. | ||||
| 1526 | The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers | ||||
| 1527 | Determination of salmeterol, -hydroxysalmeterol and fluticasone propionate in human urine and plasma for doping control using UHPLC-QTOF-MS. Biomed Chromatogr. 2021 Aug;35(8):e5114. doi: 10.1002/bmc.5114. | ||||
| 1528 | Pharmacokinetics of salmeterol and its main metabolite -hydroxysalmeterol after acute and chronic dry powder inhalation in exercising endurance-trained men: Implications for doping control. Drug Test Anal. 2021 Apr;13(4):747-761. doi: 10.1002/dta.2978. | ||||
| 1529 | The salmeterol anomaly and the need for a urine threshold. Drug Test Anal. 2022 Jun;14(6):997-1003. doi: 10.1002/dta.2810. | ||||
| 1530 | Inhibition of desipramine hydroxylation (Cytochrome P450-2D6) in vitro by quinidine and by viral protease inhibitors: relation to drug interactions in vivo. J Pharm Sci. 1998 Oct;87(10):1184-9. | ||||
| 1531 | DrugBank(Pharmacology-Metabolism)Saquinavir mesylate | ||||
| 1532 | A Phase 1 Study to Evaluate Absolute Bioavailability and Absorption, Distribution, Metabolism, and Excretion of Savolitinib in Healthy Male Volunteers | ||||
| 1533 | Instability of calcium channel antagonists during sample preparation for LC-MS-MS analysis of serum samples | ||||
| 1534 | Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis Drugs. 1996 Apr;51(4):621-38. doi: 10.2165/00003495-199651040-00007. | ||||
| 1535 | SULT 1A3 single-nucleotide polymorphism and the single dose pharmacokinetics of inhaled salbutamol enantiomers: are some athletes at risk of higher urine levels? Drug Test Anal. 2015 Feb;7(2):109-13. | ||||
| 1536 | Metabolism of sertindole: identification of the metabolites in the rat and dog, and species comparison of liver microsomal metabolism | ||||
| 1537 | Sertindole : a review of its use in schizophrenia | ||||
| 1538 | DrugBank(Pharmacology-Metabolism)Sertraline hydrochloride | ||||
| 1539 | Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos. 2005 Feb;33(2):262-70. | ||||
| 1540 | Pharmacogenetic Testing and Therapeutic Drug Monitoring Of Sertraline at a Residential Treatment Center for Children and Adolescents: A Pilot Study. Innov Pharm. 2022 Dec 26;13(4):10.24926/iip.v13i4.5035. doi: 10.24926/iip.v13i4.5035. | ||||
| 1541 | Sertraline. 2022 May 15. Drugs and Lactation Database (LactMed?) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006C. | ||||
| 1542 | Neonicotinoids and pharmaceuticals in hair of the Red fox (Vulpes vulpes) from the Cavallino-Treporti peninsula, Italy. Environ Res. 2023 Jul 1;228:115837. doi: 10.1016/j.envres.2023.115837. | ||||
| 1543 | Metabolic analysis of the illegal analogues of anti-obesity drugs using LC-Q-TOF-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2021 Jan 15;1163:122502. doi: 10.1016/j.jchromb.2020.122502. | ||||
| 1544 | In vitro biotransformation of sildenafil (Viagra): identification of human cytochromes and potential drug interactions. Drug Metab Dispos. 2000 Apr;28(4):392-7. | ||||
| 1545 | Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53 Suppl 1(Suppl 1):5S-12S. doi: 10.1046/j.0306-5251.2001.00027.x. | ||||
| 1546 | In vivo metabolic investigation of silodosin using UHPLC-QTOF-MS/MS and in silico toxicological screening of its metabolites J Mass Spectrom. 2016 Oct;51(10):867-882. doi: 10.1002/jms.3795. | ||||
| 1547 | Simvastatin Tablet, Film-Coated | ||||
| 1548 | FDA Approved Drug Products: RAPAMUNE (sirolimus) for oral use | ||||
| 1549 | Metabolism and excretion of the dipeptidyl peptidase 4 inhibitor [14C]sitagliptin in humans | ||||
| 1550 | Absorption, Distribution, Metabolism, and Excretion of [(14)C]-Sotorasib in Rats and Dogs: Interspecies Differences in Absorption, Protein Conjugation and Metabolism | ||||
| 1551 | Absorption, metabolism and excretion of [(14)C]-sotorasib in healthy male subjects: characterization of metabolites and a minor albumin-sotorasib conjugate | ||||
| 1552 | Pharmacokinetics of stiripentol in normal man: evidence of nonlinearity | ||||
| 1553 | Identification of human liver cytochrome P-450 3A4 as the enzyme responsible for fentanyl and sufentanil N-dealkylation. Anesth Analg. 1996 Jan;82(1):167-72. | ||||
| 1554 | Identification of cytochrome P450 and arylamine N-acetyltransferase isoforms involved in sulfadiazine metabolism. Drug Metab Dispos. 2005 Jul;33(7):969-76. | ||||
| 1555 | Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol. 2007 May 1;178(9):5533-42. | ||||
| 1556 | Metabolism of sulfinpyrazone sulfide and sulfinpyrazone by human liver microsomes and cDNA-expressed cytochrome P450s. Drug Metab Dispos. 2001 May;29(5):701-11. | ||||
| 1557 | Pharmacokinetics, distribution, and metabolism of [14C]sunitinib in rats, monkeys, and humans | ||||
| 1558 | Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors | ||||
| 1559 | CYP450-Mediated metabolism of suvorexant and investigation of metabolites in forensic case specimens Forensic Sci Int. 2020 Jul;312:110307. doi: 10.1016/j.forsciint.2020.110307. | ||||
| 1560 | In vitro and in vivo characterisation of the metabolism and disposition of suvorexant in humans Xenobiotica. 2016 Oct;46(10):882-95. doi: 10.3109/00498254.2015.1129565. | ||||
| 1561 | Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics Drug Metab Pharmacokinet. 2007 Oct;22(5):328-35. doi: 10.2133/dmpk.22.328. | ||||
| 1562 | Effect of Ticagrelor, a Cytochrome P450 3A4 Inhibitor, on the Pharmacokinetics of Tadalafil in Rats Pharmaceutics. 2019 Jul 20;11(7):354. doi: 10.3390/pharmaceutics11070354. | ||||
| 1563 | PharmGKB:Tamoxifen citrate | ||||
| 1564 | Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4'-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos. 2002 Aug;30(8):869-74. | ||||
| 1565 | Endoxifen and other metabolites of tamoxifen inhibit human hydroxysteroid sulfotransferase 2A1 (hSULT2A1). Drug Metab Dispos. 2014 Nov;42(11):1843-50. | ||||
| 1566 | Metabolism and transport of tamoxifen in relation to its effectiveness: new perspectives on an ongoing controversy. Future Oncol. 2014 Jan;10(1):107-22. | ||||
| 1567 | Identification and biological activity of tamoxifen metabolites in human serum Biochem Pharmacol. 1983 Jul 1;32(13):2045-52. doi: 10.1016/0006-2952(83)90425-2. | ||||
| 1568 | PharmGKB summary: tamoxifen pathway, pharmacokinetics Pharmacogenet Genomics. 2013 Nov;23(11):643-7. doi: 10.1097/FPC.0b013e3283656bc1. | ||||
| 1569 | The tamoxifen dilemma. Carcinogenesis. 1999 Jul;20(7):1153-60. | ||||
| 1570 | Pharmacokinetics and plasma protein binding of tamsulosin hydrochloride in rats, dogs, and humans Drug Metab Dispos. 1998 Mar;26(3):240-5. | ||||
| 1571 | Absorption, metabolism and excretion of tamsulosin hydrochloride in man Xenobiotica. 1996 Jun;26(6):637-45. doi: 10.3109/00498259609046739. | ||||
| 1572 | Simultaneous determination of tandospirone and its active metabolite, 1-[2-pyrimidyl]-piperazine in rat plasma by LC-MS/MS and its application to a pharmacokinetic study | ||||
| 1573 | Anti-cancer potency of tasquinimod is enhanced via albumin-binding facilitating increased uptake in the tumor microenvironment | ||||
| 1574 | Biotransformation of terodiline. III. Opposed stereoselectivity in the benzylic and aromatic hydroxylations in rat liver microsomes | ||||
| 1575 | NCIVEK- telaprevir tablet, film coated Vertex Pharmaceuticals Incorporated | ||||
| 1576 | Clinical pharmacokinetics of telithromycin, the first ketolide antibacterial. Clin Pharmacokinet. 2005;44(9):915-34. | ||||
| 1577 | Verapamil toxicity resulting from a probable interaction with telithromycin. Ann Pharmacother. 2005 Feb;39(2):357-60. | ||||
| 1578 | Pharmacokinetics and metabolism of temazepam in man and several animal species Br J Clin Pharmacol. 1979;8(1):23S-29S. doi: 10.1111/j.1365-2125.1979.tb00451.x. | ||||
| 1579 | Human liver microsomal diazepam metabolism using cDNA-expressed cytochrome P450s: role of CYP2B6, 2C19 and the 3A subfamily. Xenobiotica. 1996 Nov;26(11):1155-66. | ||||
| 1580 | American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 2511 | ||||
| 1581 | LABEL:BSRELA- tenapanor hydrochloride tablet | ||||
| 1582 | DrugBank(Pharmacology-Metabolism)Teniposide | ||||
| 1583 | PubChem:Teniposide | ||||
| 1584 | Multiple cytochrome P-450s involved in the metabolism of terbinafine suggest a limited potential for drug-drug interactions. Drug Metab Dispos. 1999 Sep;27(9):1029-38. | ||||
| 1585 | A multi-residue chiral liquid chromatography coupled with tandem mass spectrometry method for analysis of antifungal agents and their metabolites in aqueous environmental matrices. Anal Methods. 2021 Jun 14;13(22):2466-2477. doi: 10.1039/d1ay00556a. | ||||
| 1586 | Analysis of hydroxylated and N-dealkylated metabolites of terfenadine in microsomal incubates by liquid chromatography--mass spectrometry | ||||
| 1587 | Don't stay home without it | ||||
| 1588 | LABEL:TIAGABINE HYDROCHLORIDE tablet, film coated | ||||
| 1589 | Metabolism of ticagrelor in patients with acute coronary syndromes | ||||
| 1590 | DrugBank(Pharmacology-Metabolism):Ticlopidine | ||||
| 1591 | Contribution of CYP2C19 and CYP3A4 to the formation of the active nortilidine from the prodrug tilidine | ||||
| 1592 | Metabolism of ophthalmic timolol: new aspects of an old drug. Basic Clin Pharmacol Toxicol. 2011 May;108(5):297-303. | ||||
| 1593 | Quantitative determination of pentazocine in plasma and of pentazocine and metabolites in urine | ||||
| 1594 | Metabolism-mediated drug interactions associated with ritonavir-boosted tipranavir in mice. Drug Metab Dispos. 2010 May;38(5):871-8. | ||||
| 1595 | Complexity of vitamin E metabolism | ||||
| 1596 | Toremifene in postmenopausal breast cancer. Efficacy, safety and cost Drugs Aging. 1997 Oct;11(4):261-70. doi: 10.2165/00002512-199711040-00002. | ||||
| 1597 | Trends in Tramadol: Pharmacology, Metabolism, and Misuse Anesth Analg. 2017 Jan;124(1):44-51. doi: 10.1213/ANE.0000000000001683. | ||||
| 1598 | Simultaneous LC-MS/MS quantification of oxycodone, tramadol and fentanyl and their metabolites (noroxycodone, oxymorphone, O- desmethyltramadol, N- desmethyltramadol, and norfentanyl) in human plasma and whole blood collected via venepuncture and volumetric absorptive micro sampling. J Pharm Biomed Anal. 2021 Sep 5;203:114171. doi: 10.1016/j.jpba.2021.114171. | ||||
| 1599 | Pharmacokinetics of trazodone and its major metabolite m-chlorophenylpiperazine in plasma and brain of rats Int J Neuropsychopharmacol. 1999 Mar;2(1):17-23. doi: 10.1017/S1461145799001303. | ||||
| 1600 | Retinoic acid metabolism and mechanism of action: a review | ||||
| 1601 | A mass balance study to evaluate the biotransformation and excretion of [14C]-triamcinolone acetonide following oral administration J Clin Pharmacol. 2000 Jul;40(7):770-80. doi: 10.1177/00912700022009413. | ||||
| 1602 | Metabolic fate of a synthetic corticosteroid (triamcinolone) in the dog J Biol Chem. 1961 Apr;236:1038-42. | ||||
| 1603 | Triazolam disposition Clin Pharmacol Ther. 1981 Jan;29(1):81-93. doi: 10.1038/clpt.1981.14. | ||||
| 1604 | Triazolam substrate inhibition: evidence of competition for heme-bound reactive oxygen within the CYP3A4 active site. Drug Metab Dispos. 2001 Jan;29(1):70-5. | ||||
| 1605 | Total determination of triclabendazole and its metabolites in bovine tissues using liquid chromatography-tandem mass spectrometry J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Mar 1;1109:54-59. doi: 10.1016/j.jchromb.2019.01.021. | ||||
| 1606 | Egaten FDA label | ||||
| 1607 | LABEL:KLIEF- trifarotene cream | ||||
| 1608 | Trimethadione metabolism by human liver cytochrome P450: evidence for the involvement of CYP2E1. Xenobiotica. 1998 Nov;28(11):1041-7. | ||||
| 1609 | In Vitro Hepatic Oxidative Biotransformation of Trimethoprim Drug Metab Dispos. 2015 Sep;43(9):1372-80. doi: 10.1124/dmd.115.065193. | ||||
| 1610 | Oxidation of troglitazone to a quinone-type metabolite catalyzed by cytochrome P-450 2C8 and P-450 3A4 in human liver microsomes. Drug Metab Dispos. 1999 Nov;27(11):1260-6. | ||||
| 1611 | Intestinal motility and its disorders Scand J Urol Nephrol Suppl. 1992;142:18-21. | ||||
| 1612 | Inactivation of cytochrome P-450 by a troleandomycin metabolite. Protective role of glutathione J Pharmacol Exp Ther. 1983 Mar;224(3):685-91. | ||||
| 1613 | Pharmacokinetics and metabolism of the 5-hydroxytryptamine antagonist tropisetron after single oral doses in humans | ||||
| 1614 | Cytochrome P450 3A4 is the major enzyme responsible for the metabolism of laquinimod, a novel immunomodulator. Drug Metab Dispos. 2005 Jun;33(6):866-72. | ||||
| 1615 | Pharmacokinetics, toxicological and clinical aspects of ulipristal acetate: insights into the mechanisms implicated in the hepatic toxicity Drug Metab Rev. 2021 Aug;53(3):375-383. doi: 10.1080/03602532.2021.1917599. | ||||
| 1616 | LABEL:INVOQ- upadacitinib tablet, extended release | ||||
| 1617 | Pharmacokinetics of vandetanib: three phase I studies in healthy subjects Clin Ther. 2012 Jan;34(1):221-37. doi: 10.1016/j.clinthera.2011.11.011. | ||||
| 1618 | Functional evaluation of vandetanib metabolism by CYP3A4 variants and potential drug interactions in vitro Chem Biol Interact. 2021 Dec 1;350:109700. doi: 10.1016/j.cbi.2021.109700. | ||||
| 1619 | Identification of Human Enzymes Oxidizing the Anti-Thyroid-Cancer Drug Vandetanib and Explanation of the High Efficiency of Cytochrome P450 3A4 in its Oxidation Int J Mol Sci. 2019 Jul 10;20(14):3392. doi: 10.3390/ijms20143392. | ||||
| 1620 | Vardenafil preclinical trial data: potency, pharmacodynamics, pharmacokinetics, and adverse events Int J Impot Res. 2004 Jun;16 Suppl 1:S34-7. doi: 10.1038/sj.ijir.3901213. | ||||
| 1621 | High-performance liquid chromatographic method with amperometric detection employing boron-doped diamond electrode for the determination of sildenafil, vardenafil and their main metabolites in plasma J Chromatogr A. 2011 Nov 4;1218(44):7996-8001. doi: 10.1016/j.chroma.2011.09.001. | ||||
| 1622 | A simple and sensitive HPLC-FL method for bioanalysis of velpatasvir, a novel hepatitis C virus NS5A inhibitor, in rat plasma: Investigation of factors determining its oral bioavailability J Chromatogr B Analyt Technol Biomed Life Sci. 2022 Oct 1;1208:123399. doi: 10.1016/j.jchromb.2022.123399. | ||||
| 1623 | Novel rapid liquid chromatography tandem masspectrometry method for vemurafenib and metabolites in human plasma, including metabolite concentrations at steady state Biomed Chromatogr. 2016 Aug;30(8):1234-9. doi: 10.1002/bmc.3672. | ||||
| 1624 | O- and N-demethylation of venlafaxine in vitro by human liver microsomes and by microsomes from cDNA-transfected cells: effect of metabolic inhibitors and SSRI antidepressants. Neuropsychopharmacology. 1999 May;20(5):480-90. | ||||
| 1625 | Venlafaxine oxidation in vitro is catalysed by CYP2D6. Br J Clin Pharmacol. 1996 Feb;41(2):149-56. | ||||
| 1626 | Comparison of the pharmacokinetics of venlafaxine extended release and desvenlafaxine in extensive and poor cytochrome P450 2D6 metabolizers | ||||
| 1627 | Accidental intoxications in toddlers: lack of cross-reactivity of vilazodone and its urinary metabolite M17 with drug of abuse screening immunoassays | ||||
| 1628 | DETERMINATION OF VENLAFAXINE, VILAZODONE AND THEIR MAIN ACTIVE METABOLITES IN HUMAN SERUM BY HPLC-DAD AND HPLC-MS | ||||
| 1629 | Pharmacokinetics and metabolism of vinblastine in humans | ||||
| 1630 | Selective metabolism of vincristine in vitro by CYP3A5. Drug Metab Dispos. 2006 Aug;34(8):1317-27. | ||||
| 1631 | A single dose mass balance study of the Hedgehog pathway inhibitor vismodegib (GDC-0449) in humans using accelerator mass spectrometry | ||||
| 1632 | Absorption, distribution, metabolism, and excretion of [1?C]GDC-0449 (vismodegib), an orally active hedgehog pathway inhibitor, in rats and dogs: a unique metabolic pathway via pyridine ring opening | ||||
| 1633 | Preclinical assessment of the absorption, distribution, metabolism and excretion of GDC-0449 (2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide), an orally bioavailable systemic Hedgehog signalling pathway inhibitor | ||||
| 1634 | Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date | ||||
| 1635 | Identification of human liver cytochrome P450 enzymes involved in the metabolism of SCH 530348 (Vorapaxar), a potent oral thrombin protease-activated receptor 1 antagonist | ||||
| 1636 | The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human | ||||
| 1637 | Vortioxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2018 Jun;57(6):673-686. | ||||
| 1638 | FDA Approved Drug Products:Oxbryta (voxelotor) tablets | ||||
| 1639 | Comparative pharmacokinetics of vitamin K antagonists: warfarin, phenprocoumon and acenocoumarol. Clin Pharmacokinet. 2005;44(12):1227-46. | ||||
| 1640 | BioTransformer: a comprehensive computational tool for small molecule metabolism prediction and metabolite identification | ||||
| 1641 | Metabolism and excretion of zafirlukast in dogs, rats, and mice | ||||
| 1642 | Variability of Zaleplon 5-Oxidase Activity in Mice and Humans, and Inhibition by Raloxifene | ||||
| 1643 | Separation and identification of zaleplon metabolites in human urine using capillary electrophoresis with laser-induced fluorescence detection and liquid chromatography-mass spectrometry | ||||
| 1644 | Metabolism and pharmacokinetics of the combination Zidovudine plus Lamivudine in the adult Erythrocebus patas monkey determined by liquid chromatography-tandem mass spectrometric analysis | ||||
| 1645 | Metabolism and excretion of a new antipsychotic drug, ziprasidone, in humans | ||||
| 1646 | [Limits of jaw orthopedic treatment] | ||||
| 1647 | Antiprogestin pharmacodynamics, pharmacokinetics, and metabolism: implications for their long-term use. J Pharmacokinet Biopharm. 1997 Dec;25(6):647-72. | ||||
| 1648 | Zolpidem urine excretion profiles and cross-reactivity with ELISA(?) kits in subjects using Zolpidem or Ambien(?) CR as a prescription sleep aid | ||||
| 1649 | Qualitative analysis of 7- and 8-hydroxyzolpidem and discovery of novel zolpidem metabolites in postmortem urine using liquid chromatography-tandem mass spectrometry | ||||
| 1650 | Zonisamide: review of pharmacology, clinical efficacy, tolerability, and safety | ||||
| 1651 | Distribution of zopiclone and main metabolites in hair following a single dose | ||||
| 1652 | Norzotepine, a major metabolite of zotepine, exerts atypical antipsychotic-like and antidepressant-like actions through its potent inhibition of norepinephrine reuptake | ||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

