| General Information of This Metabolic Reaction (MR) (ID:
MR001185) |
| Formula |
|
| Reactant |
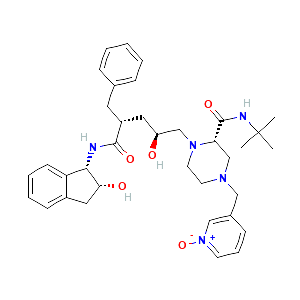
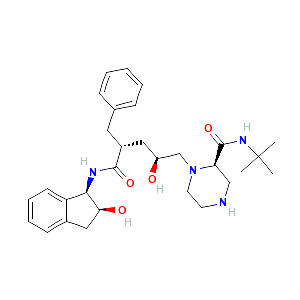
Indinavir sulfate metabolite M4a |
Product |
Indinavir sulfate metabolite M6 |
|
Reactant Info
|
Product Info
|
|
Metabolic Enzyme
|
Cytochrome P450 3A4 (CYP3A4)
|
DME Info
|
|
Metabolic Type
|
Oxidation
-
N-Depyridomethylation
|
|
|
|
|
|
|
|
| Other MR(s) Related to The Reactant of This MR |
|
Other MR(s) That Produce The Reactant of This MR
|
|
|
|
Other MR(s) That Metabolize The Reactant of This MR
|
|
|
| Other MR(s) Related to The Product of This MR |
|
Other MR(s) That Produce The Product of This MR
|
|
|
|
Other MR(s) That Metabolize The Produtc of This MR
|
|
|
| References |
| 1 |
Hepatic and intestinal metabolism of indinavir, an HIV protease inhibitor, in rat and human microsomes. Major role of CYP3A. Biochem Pharmacol. 1997 Apr 25;53(8):1187-95.
|
| 2 |
Involvement of CYP3A4 and MDR1 in altered metabolism and transport of indinavir in 1,25(OH)(2)D(3)-treated Caco-2 cells. Eur J Pharm Sci. 2023 Apr 1;183:106396. doi: 10.1016/j.ejps.2023.106396.
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.