| References |
| 1 |
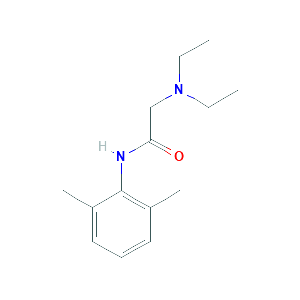
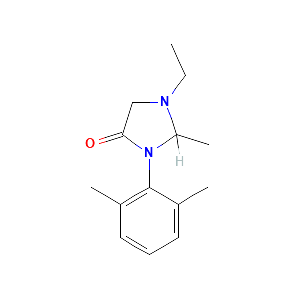
Involvement of CYP1A2 and CYP3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab Dispos. 2000 Aug;28(8):959-65.
|
| 2 |
Quantification of lidocaine and several metabolites utilizing chemical-ionization mass spectrometry and stable isotope labeling J Pharm Sci. 1977 Aug;66(8):1180-90. doi: 10.1002/jps.2600660834.
|
| 3 |
An innovative nanoparticle-modified carbon paste sensor for ultrasensitive detection of lignocaine and its extremely carcinogenic metabolite residues in bovine food samples: Application of NEMI, ESA, AGREE, ComplexGAPI, and RGB12 algorithms. Food Chem. 2023 Jun 12;426:136579. doi: 10.1016/j.foodchem.2023.136579.
|
| 4 |
In vitro metabolism of lidocaine in subcellular post-mitochondrial fractions and precision cut slices from cattle liver. Toxicol In Vitro. 2021 Oct;76:105228. doi: 10.1016/j.tiv.2021.105228.
|
| 5 |
The Use and Method of Action of Intravenous Lidocaine and Its Metabolite in Headache Disorders Headache. 2018 May;58(5):783-789. doi: 10.1111/head.13298.
|