| General Information of This Metabolic Reaction (MR) (ID:
MR001551) |
| Formula |
|
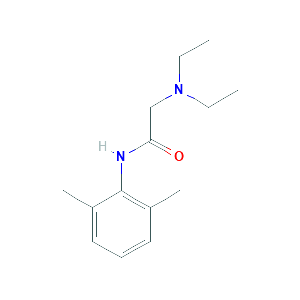
| Reactant |
Lidocaine |
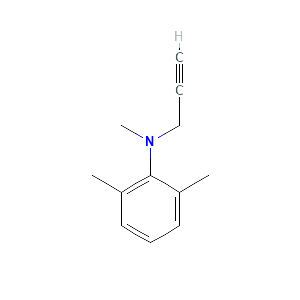
Product |
2,6-xylidine |
|
Reactant Info
|
Product Info
|
|
|
|
|
|
|
|
| Other MR(s) Related to The Reactant of This MR |
|
Other MR(s) That Metabolize The Reactant of This MR
|
|
|
| Other MR(s) Related to The Product of This MR |
|
Other MR(s) That Produce The Product of This MR
|
|
|
|
Other MR(s) That Metabolize The Produtc of This MR
|
|
|
| References |
| 1 |
Involvement of CYP1A2 and CYP3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab Dispos. 2000 Aug;28(8):959-65.
|
| 2 |
The Use and Method of Action of Intravenous Lidocaine and Its Metabolite in Headache Disorders Headache. 2018 May;58(5):783-789. doi: 10.1111/head.13298.
|
| 3 |
Metabolism of tocainide in the rat
|
| 4 |
Quantification of lidocaine and several metabolites utilizing chemical-ionization mass spectrometry and stable isotope labeling J Pharm Sci. 1977 Aug;66(8):1180-90. doi: 10.1002/jps.2600660834.
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.