| General Information of This Metabolic Reaction (MR) (ID:
MR001877) |
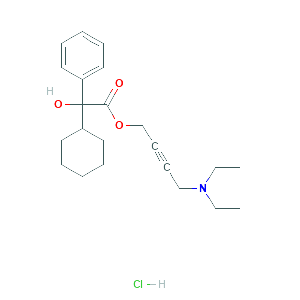
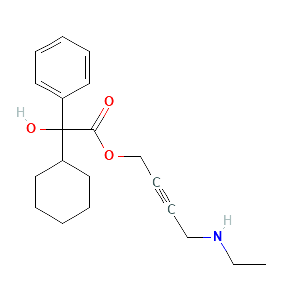
| Formula |
|
| Reactant |
Oxybutynin chloride |
Product |
N-desethyloxybutynin |
|
Reactant Info
|
Product Info
|
|
Metabolic Enzyme
|
Cytochrome P450 3A4 (CYP3A4)
|
DME Info
|
|
Cytochrome P450 3A5 (CYP3A5)
|
DME Info
|
|
Metabolic Type
|
Oxidation
-
N-Deethylation
|
|
|
|
|
|
|
|
| Other MR(s) Related to The Product of This MR |
|
Other MR(s) That Produce The Product of This MR
|
|
|
|
Other MR(s) That Metabolize The Produtc of This MR
|
|
|
| References |
| 1 |
New insights in the metabolism of oxybutynin: evidence of N-oxidation of propargylamine moiety and rearrangement to enaminoketone
|
| 2 |
Oxybutynin. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability Drugs Aging. 1995 Mar;6(3):243-62. doi: 10.2165/00002512-199506030-00007.
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.