| References |
| 1 |
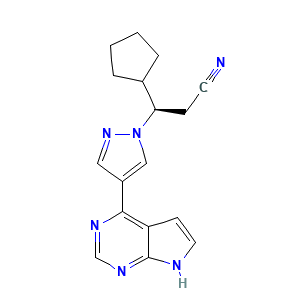
Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans Drug Metab Dispos. 2010 Nov;38(11):2023-31. doi: 10.1124/dmd.110.033787.
|
| 2 |
The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers
|
| 3 |
Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675.
|
| 4 |
DrugBank(Pharmacology-Metabolism):Ruxolitinib
|