| General Information of This Metabolic Reaction (MR) (ID:
MR013369) |
| Formula |
|
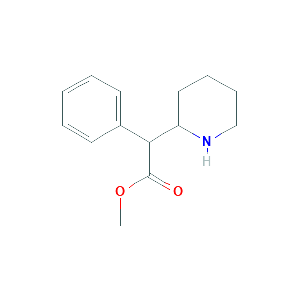
| Reactant |
Methylphenidate |
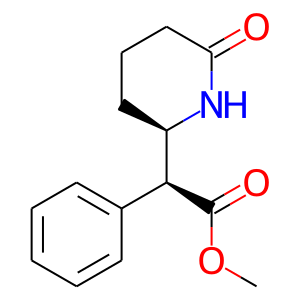
Product |
6-Oxo-MPH |
|
Reactant Info
|
Product Info
|
|
|
|
|
|
|
|
| Other MR(s) Related to The Reactant of This MR |
|
Other MR(s) That Metabolize The Reactant of This MR
|
|
|
| Other MR(s) Related to The Product of This MR |
|
Other MR(s) That Metabolize The Produtc of This MR
|
|
|
| References |
| 1 |
Metabolism of methylphenidate in dog and rat
|
| 2 |
Methylphenidate is stereoselectively hydrolyzed by human carboxylesterase CES1A1. J Pharmacol Exp Ther. 2004 Aug;310(2):469-76.
|
| 3 |
Metabolomics of Methylphenidate and Ethylphenidate: Implications in Pharmacological and Toxicological Effects
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.