| General Information of Drug (ID:
DR1054) |
| Drug Name |
Methylphenidate
|
| Synonyms |
Meridil; Metadate; Methyl phenidate; Methyl phenidyl acetate; Methyl phenidylacetate; Methylfenidan; Methylin; Methylphenidan; Methylphenidate HCl; Methylphenidatum; Methylphenidatum [INN-Latin]; Metilfenidato; Metilfenidato [INN-Spanish]; Metilfenidato [Italian]; Phenidylate; Plimasine; Ritalin; d-methylphenidate HCl; methylphenidate; Calocain; Concerta; Daytrana; 113-45-1; 4311/B Ciba; Methyl alpha-phenyl-alpha-(2-piperidyl)acetate; NCI-C56280; alpha-Phenyl-2-piperidineacetic acid methyl ester; methyl phenyl(piperidin-2-yl)acetate
|
| Indication |
Attention deficit hyperactivity disorder
[ICD11: 6A05]
|
Approved
|
[1]
|
| Structure |
|
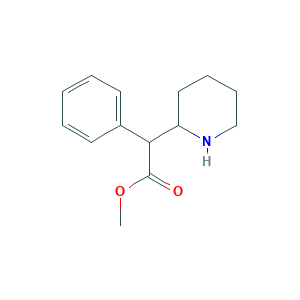 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
233.31 |
Topological Polar Surface Area |
38.3 |
| Heavy Atom Count |
17 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 4158
- PubChem SID
-
9405
; 5336253
; 7979945
; 8152600
; 10504398
; 14798219
; 26746622
; 29223265
; 46505929
; 47206727
; 48416258
; 49854760
; 53789341
; 57322165
; 78286794
; 85209963
; 103209574
; 103858000
; 103958410
; 104305599
; 124954808
; 127787857
; 134337354
; 134974024
; 135709935
; 136897384
; 137007933
; 142361369
; 160963768
; 163411030
; 178103810
; 179148478
; 216106193
; 223897828
; 226423048
; 249807024
; 249948308
; 252404612
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02PPN
- Formula
- C14H19NO2
- Canonical SMILES
- COC(=O)C(C1CCCCN1)C2=CC=CC=C2
- InChI
- 1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3
- InChIKey
- DUGOZIWVEXMGBE-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


