| General Information of Drug (ID:
DR5092) |
| Drug Name |
Spironolactone
|
| Synonyms |
Abbolactone; Acelat; Aldace; Aldactazide; Aldactide; Aldactone; Alderon; Aldopur; Almatol; Altex; Aquareduct; Deverol; Diatensec; Dira; Duraspiron; Espironolactona; Euteberol; Flumach; Frumikal; Jenaspiron; Lacalmin; Lacdene; Laractone; Melarcon; Nefurofan; NovoSpiroton; Osyrol; Practon; SNL; Sagisal; Sincomen; Spiractin; Spiresis; Spiretic; Spiridon; Spirobeta; Spiroctan; Spiroctanie; Spiroderm; Spirogamma; Spirolactone; Spirolakton; Spirolang; Spirolone; Spirone; Spironocompren; Spironolactonum; Spironolattone; Spironone; Spirospare; Sprioderm; Suracton; Uractone; Urusonin; Veroshpiron; Verospiron; Verospirone; Xenalon; Aldactone A; Alphapharm Brand of Spironolactone; Alpharma Brand of Spironolactone; Alter Brand of Spironolactone; Ashbourne Brand of Spironolactone; Azupharma Brand of Spironolactone; Betapharm Brand of Spironolactone; Cardel Brand of Spironolactone; Ct Arzneimittel Brand of Spironolactone; Dexo Brand of Spironolactone; Espironolactona Alter; Espironolactona Mundogen; Generosan Brand of Spironolactone; Hormosan Brand of Spironolactone; Jenapharm Brand of Spironolactone; Merck dura Brand of Spironolactone; Mundogen Brand of Spironolactone; Novo Spiroton; Novopharm Brand of Spironolactone; Pfizer Brand of Spironolactone; Pharmafrid Brand of Spironolactone; Roche Brand of Spironolactone; Searle Brand of Spironolactone; Spiro von ct; Spirono Isis; Spironolactone A; Spironolattone [DCIT]; Verospirone Opianin; Worwag Brand of Spironolactone; LT00772287; SC 9420; SC9420; Aldactazide (TN); Aldactone (TN); Berlactone (TN); Ct-Arzneimittel Brand of Spironolactone; Espironolactona [INN-Spanish]; Mayoly-Spindler Brand of Spironolactone; Novo-Spiroton; SC-9420; Spiractin (TN); Spiro-Tablinen; Spirono-Isis; Spironolactonum [INN-Latin]; Spirotone (TN); Supra-puren; Verospiron (TN); Von ct, spiro; Novo-Spiroton (TN); Spironolactone [BAN:INN:JAN]; Spironolactone [INN:BAN:JAN]; Spiro L.U.T.; Spironolactone (JP15/USP/INN); Spiro[17H-cyclopenta[a]phenauthrene-17,2'-(3'H)-furan]; Spiro(17H-cyclopenta(a)phenauthrene-17,2'-(3'H)-furan); 4-Pregnen-21-oic acid-17alpha-ol-3-one-7alpha-thiol gamma-lactone 7-acetate; 7-alpha-Acetylthio-3-oxo-17-alpha-pregn-4-ene-21,17-beta-carbolactone; 7alpha-(acetylsulfanyl)-3-oxo-17alpha-pregn-4-ene-21,17-carbolactone
|
| Indication |
Congestive heart failure
[ICD11: BD10-BD1Z]
|
Approved
|
[1]
|
| Structure |
|
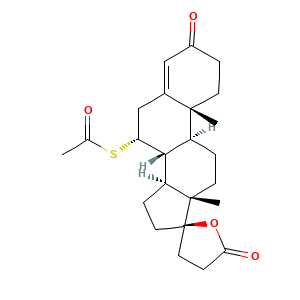 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
416.6 |
Topological Polar Surface Area |
85.7 |
| Heavy Atom Count |
29 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 5833
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0EP0C
- Formula
- C24H32O4S
- Canonical SMILES
- CC(=O)S[C@@H]1CC2=CC(=O)CC[C@@]2([C@@H]3[C@@H]1[C@@H]4CC[C@]5([C@]4(CC3)C)CCC(=O)O5)C
- InChI
- InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1
- InChIKey
- LXMSZDCAJNLERA-ZHYRCANASA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


