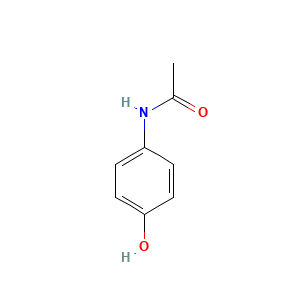
Details of the Drug Metabolite (DM)
| Toxicity Properties of This DM | ||||||
|---|---|---|---|---|---|---|
| Documented Toxicity Properties | ||||||
| Toxicity Class | Toxic | PMID: 34785186 | ||||
| Predicted Toxicity Properties | ||||||
| Physical and chemical properties |
LogP
The log of the n-octanol/water distribution coefficient. LogP possess a leading position with considerable impact on both membrane permeability and hydrophobic binding to macromolecules. Therefore, LogP is widely used in drug discovery and development as an indicator of potential utility of a solute as a drug. The predicted logP of a compound in the range from 0 to 3 log mol/L will be considered proper. |
0.608 |
TPSA
Topological polar surface area In TPSA, PSA is estimated only from the syntype (topology) of atoms in the molecule, without considering the three-dimensional structure of the molecule, which is the origin of the name topological polar surface area. The TPSA of a compound in the range from 0 to 140 will be considered proper, based on Veber rule. |
49.33 | ||
|
Pfizer Rule: Accepted
Molecules with a high log P (>3) and low TPSA (<75) are likely to be toxic. Pfizer infered the relationship between the physicochemical properties and toxicity of the drug from an animal tolerability (IVT) study dataset of 245 preclinical Pfizer compounds.Compounds with a high log P (>3) and low TPSA ( <75) are likely to be toxic. |
||||||
| Structural Characteristics |
ALARM NMR Rule
Molecules containing the reactivity-related thiol substructures are likely to be toxic. The high-throughput screening (HTS) hit rate of reactive compounds was evaluated by NMR screening, X-ray crystallography and other biochemical and biophysical experiments, and then 75 thiol substructures for predicting reactivity were obtained by computational means for 2348 screening hit reactive compounds and 1156 reactive compounds obtained by La protein experiments.The molecule was matched to 75 reactivity-related substructures to obtain the information how many alarm groups the molecule contained and determine whether it was a thiol-reactive compound. Molecules with the thiol substructures are likely to be toxic. |
2 |
PAINS
Molecules containing the reactive substructures are likely to be toxic. Pan Assay Interference Compounds (PAINS) is one of the most famous frequent hitters filters, which comprises 480 substructures derived from the analysis of FHs determined by six target-based HTS assay. By application of these filters, it is easier to screen false positive hits and to flag suspicious compounds in screening databases. One of the most authoritative medicine magazines Journal of Medicinal Chemistry even requires authors to provide the screening results with the PAINS alerts of active compounds when submitting manuscripts. |
0 | ||
|
BMS Rule
Molecules containing the reactivity-related substructures are likely to be toxic. BMS's primary HTS data over the past 12 years was evaluated and analyzed to determine the correlation of a group of compound functional groups with Promiscuity, defined as a drug that acts with multiple molecular targets and exhibits different pharmacological effects. |
0 |
Chelator Rule
Molecules containing the substructures associated with metalloprotease targeting are likely to be toxic. The chelate substructure fragment library (eCFL) for targeting metalloproteinases was prepared and its effectiveness in screening metalloproteinase inhibitors was verified by analysis and fluorescence-based assay experiments, and 55 substructures associated with metalloprotease targeting were finally determined as alert structures. |
0 | |||
|
Genotoxic Carcinogenicity Rule
Molecules containing the Genotoxic substructures are likely to be carcinogenic. By constructing a molecular structure dataset containing the corresponding Ames test data (mutagens and non-mutagens). The substructure of the dataset is searched, and then the toxic substructure obtained by using chemical and mechanical knowledge and statistical criteria is derived, and the new toxic substructure is obtained and approved, and finally the reliability of the verification set is verified. Molecules containing these substructures may cause carcinogenicity or mutagenicity through genotoxic mechanisms.There are 117 substructures in this endpoint. |
3 |
Non-genotoxic Carcinogenicity Rule
Molecules containing the NonGenotoxic substructures are likely to be carcinogenic. Through the analysis and verification of the existing molecular library or the molecular library mined by data, the list of non-gene carcinogenic substructures (SA) is obtained according to the computerized data mining analysis, and finally the reliability of the substructure is verified. Molecules containing these substructures may cause carcinogenicity through nongenotoxic mechanisms. There are 23 substructures in this endpoint. |
0 | |||
| Toxicity Model Prediction |
hERG Blockers
The possibility of causing cardiotoxicity. The human ether-a-go-go related gene. The During cardiac depolarization and repolarization, a voltage-gated potassium channel encoded by hERG plays a major role in the regulation of the exchange of cardiac action potential and resting potential. The hERG blockade may cause long QT syndrome (LQTS), arrhythmia, and Torsade de Pointes (TdP), which lead to palpitations, fainting, or even sudden death.So build a model by collecting a dataset to predict whether a compound is a hERG Blocker. The output value is the probability of being toxic, within the range of 0 to 1. 0-0.3: excellent; 0.3-0.7: medium; 0.7-1.0: poor. |
0.02 (---) |
H-HT
The possibility of causing .hepatotoxicity. The human hepatotoxicity. Drug induced liver injury is of great concern for patient safety and a major cause for drug withdrawal from the market. Adverse hepatic effects in clinical trials often lead to a late and costly termination of drug development programs.So build a model by collecting datasets to predict whether compounds will cause hepatotoxicity. The output value is the probability of being toxic, within the range of 0 to 1. 0-0.3: excellent; 0.3-0.7: medium; 0.7-1.0: poor. |
0.18 (--) | ||
|
DILI
The possibility of causing liver injury. Drug-induced liver injury (DILI) has become the most common safety problem of drug withdrawal from the market over the past 50 years.So build a model by collecting datasets to predict whether compounds will cause liver injury. The output value is the probability of being toxic, within the range of 0 to 1. 0-0.3: excellent; 0.3-0.7: medium; 0.7-1.0: poor. |
0.385 (-) |
CAMES Toxicity
The possibility of causing mutagenicity. The Ames test for mutagenicity. The mutagenic effect has a close relationship with the carcinogenicity, and it is the most widely used assay for testing the mutagenicity of compounds.So build a model by collecting datasets to predict whether compounds will cause mutagenicity. The output value is the probability of being toxic, within the range of 0 to 1. 0-0.3: excellent; 0.3-0.7: medium; 0.7-1.0: poor. |
0.321 (-) | |||
|
Carcinogencity
The possibility of causing Carcinogencity. Among various toxicological endpoints of chemical substances, carcinogenicity is of great concern because of its serious effects on human health. The carcinogenic mechanism of chemicals may be due to their ability to damage the genome or disrupt cellular metabolic processes. Many approved drugs have been identified as carcinogens in humans or animals and have been withdrawn from the market.So build a model by collecting datasets to predict whether compounds will cause Carcinogencity. The output value is the probability of being toxic, within the range of 0 to 1. 0-0.3: excellent; 0.3-0.7: medium; 0.7-1.0: poor. |
0.624 (+) |
Respiratory Toxicity
The possibility of causing Respiratory Toxicity. Among these safety issues, respiratory toxicity has become the main cause of drug withdrawal. Drug-induced respiratory toxicity is usually underdiagnosed because it may not have distinct early signs or symptoms in common medications and can occur with significant morbidity and mortality.Therefore, careful surveillance and treatment of respiratory toxicity is of great importance.So build a model by collecting datasets to predict whether compounds will cause Respiratory Toxicity. The output value is the probability of being toxic, within the range of 0 to 1. 0-0.3: excellent; 0.3-0.7: medium; 0.7-1.0: poor. |
0.039 (---) | |||
| Full List of Drug-Metabolizing Enzyme (DME) Related to This DM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DME(s) Producing This DM through Metabolism | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DME(s) Metabolizing This DM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Full List of Drug(s) That Produce This DM By Metabolism | ||||||
|---|---|---|---|---|---|---|
| Phenazopyridine | DR2301 | Phase 4 | Infectious cystitis | |||
| BCP-13498 | DR1359 | Phase 2 | Anaesthesia | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.