| Synonyms |
Fluoroblastin; Fluoroplex; Fluorouracilo; Fluorouracilum; Fluracil; Fluracilum; Fluril; Fluro Uracil; Fluroblastin; Ftoruracil; 5-Fluorouracil; Kecimeton; Phthoruracil; Phtoruracil; Queroplex; Timazin; fluorouracil; 2,4(1H,3H)-Pyrimidinedione, 5-fluoro-; 2,4-Dihydroxy-5-fluoropyrimidine; 5 Fluorouracil; 5-FU; 5-Fluoracil; 5-Fluoro-2,4(1H,3H)-pyrimidinedione; 5-fluoro-1H-pyrimidine-2,4-dione; 5-fluoropyrimidine-2,4(1H,3H)-dione; 51-21-8; Carac; Fluri; Ulup; Adrucil; Arumel; Carzonal; Effluderm (free base); Efudex; Efudix; Efurix
|
| Cross-matching ID |
- PubChem CID
- 3385
- PubChem SID
-
9851
; 82653
; 595836
; 603131
; 841046
; 3139714
; 5132902
; 5367838
; 7847650
; 7891022
; 7978600
; 8139872
; 8149350
; 8152156
; 10524722
; 11111190
; 11335229
; 11360468
; 11363735
; 11366297
; 11368859
; 11371368
; 11374392
; 11377021
; 11406045
; 11461440
; 11484027
; 11487892
; 11490250
; 11492372
; 11494655
; 11538022
; 15218968
; 17389875
; 17405099
; 22391543
; 24276773
; 24278439
; 24871165
; 24894963
; 25346604
; 25621761
; 26611750
; 26679238
; 26697058
; 26747342
; 26747343
; 26747344
; 26752979
; 26758708
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05LEO
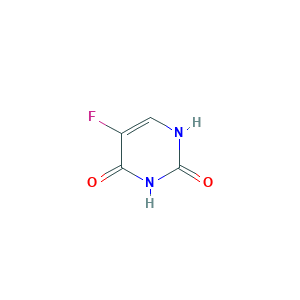
- Formula
- C4H3FN2O2
- Canonical SMILES
- C1=C(C(=O)NC(=O)N1)F
- InChI
- 1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
- InChIKey
- GHASVSINZRGABV-UHFFFAOYSA-N
|