Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0103) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Amoxapine
|
|||||
| Synonyms |
Amoxan; Amoxapina; Amoxapina [INN-Spanish]; Amoxapinum; Amoxapinum [INN-Latin]; Amoxepine; Ascendin; Asendin; Asendis; Demolox; Desmethylloxapin; Moxadil; amoxapine; 14028-44-5; 2-Chlor-11-(1-piperazinyl)dibenz(b,f)(1,4)oxazepin; 2-Chloro-11-(1-piperazinyl)dibenz(b,f)(1,4)oxazepine; 2-Chloro-11-(1-piperazinyl)dibenz[b,f][1,4]oxazepine; BRN 0832057; C17H16ClN3O; CHEMBL1113; CL 67772; CL-67772; Dibenz[b,f][1,4]oxazepine, 2-chloro-11-(1-piperazinyl)-; EINECS 237-867-1; MFCD00069210; MLS000069371; UNII-R63VQ857OT
|
|||||
| Indication | Depression [ICD11: 6A71] | Approved | [1] | |||
| Structure |
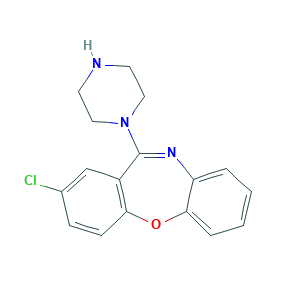 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 313.8 | Topological Polar Surface Area | 36.9 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

