Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0125) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Aprepitant
|
|||||
| Synonyms |
Aprepitant; L 754030; L-754030; MK 0869; MK 869; MK-0869; MK-869; MK0869; aprepitantum; 170729-80-3; 1NF15YR6UY; 5-[[(2R,3S)-2-[(1R)-1-[3,5-BIS(Trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one; 5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholin-4-yl]methyl]-1,2-dihydro-1,2,4-triazol-3-one; CHEBI:499361; CHEMBL1471; Emend; UNII-1NF15YR6UY
|
|||||
| Indication | Functional nausea/vomiting [ICD11: DD90] | Approved | [1] | |||
| Structure |
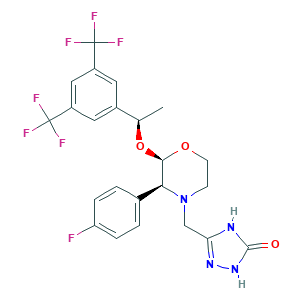 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 534.4 | Topological Polar Surface Area | 75.2 | ||
| Heavy Atom Count | 37 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 12 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Aprepitant was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos. 2004 Nov;32(11):1287-92. | |||||
| 3 | Lack of effect of aprepitant on the pharmacokinetics of docetaxel in cancer patients. Cancer Chemother Pharmacol. 2005 Jun;55(6):609-16. | |||||
| 4 | In vitro glucuronidation of aprepitant: a moderate inhibitor of UGT2B7 Xenobiotica. 2015;45(11):990-8. doi: 10.3109/00498254.2015.1038743. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

