| General Information of Drug (ID:
DR0188) |
| Drug Name |
Bendamustine
|
| Synonyms |
Bendamustina; Bendamustina [Spanish]; Bendamustine; Bendamustine (INN); Bendamustine [INN]; Bendamustinum; Bendamustinum [Latin]; bendamustin; 16506-27-7; 1H-Benzimidazole-2-butanoic acid, 5-(bis(2-chloroethyl)amino)-1-methyl-; 4-(5-(Bis(2-chloroethyl)amino)-1-methyl-1H-benzo[d]imidazol-2-yl)butanoic acid; 4-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid; 9266D9P3PQ; DSSTox_CID_26888; DSSTox_GSID_46888; DSSTox_RID_81991; NCGC00181170-01; UNII-9266D9P3PQ
|
| Indication |
Chronic lymphocytic leukaemia
[ICD11: 2A82]
|
Approved
|
[1]
|
| Structure |
|
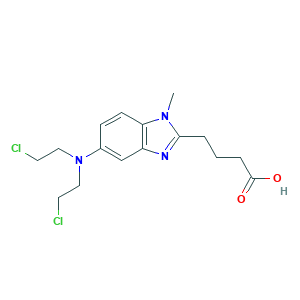 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
358.3 |
Topological Polar Surface Area |
58.4 |
| Heavy Atom Count |
23 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 65628
- PubChem SID
-
590536
; 6267839
; 8189814
; 14901248
; 43122399
; 49882625
; 50064534
; 50125886
; 51091826
; 53790479
; 57315623
; 77194776
; 103590063
; 104334244
; 125093188
; 126616720
; 126646730
; 126669991
; 135021598
; 135200933
; 136946484
; 137006822
; 143108248
; 144115692
; 144206324
; 163305696
; 170465340
; 174007431
; 175267866
; 176225504
; 176484647
; 176484951
; 179322551
; 187051781
; 196111115
; 198993387
; 215780746
; 224321241
; 226414200
; 242059805
; 244970987
; 249862320
; 250201937
; 251899454
; 251916785
; 251918024
; 252076446
; 252345440
; 252416248
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01CYA
- Formula
- C16H21Cl2N3O2
- Canonical SMILES
- CN1C2=C(C=C(C=C2)N(CCCl)CCCl)N=C1CCCC(=O)O
- InChI
- 1S/C16H21Cl2N3O2/c1-20-14-6-5-12(21(9-7-17)10-8-18)11-13(14)19-15(20)3-2-4-16(22)23/h5-6,11H,2-4,7-10H2,1H3,(H,22,23)
- InChIKey
- YTKUWDBFDASYHO-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


