| Synonyms |
Gloriosine; N-Deacetyl-N-formyl Colchicine; N-Deacetyl-N-formylcolchicine; N-Formyl-Deacetylcolchicine; N-Formyl-N-deacetylcolchicine; Formyldescaetylcolchicine, N-; N-Formyldeacetylcolchicine; N-Formyldesacetylcolchicine; U02803H7OJ; AC1L2N2A; BRN 2824080; CHEMBL85710; COLCHICINE, N-DEACETYL-N-FORMYL-; Formamide, N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)-, (S)-; N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]formamide; NSC 403142; NSC403142; UNII-U02803H7OJ
|
| Cross-matching ID |
- PubChem CID
- 23890
- PubChem SID
-
473586
; 8141688
; 8167432
; 26756917
; 29291001
; 49955736
; 57331490
; 77548941
; 84981402
; 103291345
; 104359407
; 124892459
; 134987857
; 135626447
; 162259585
; 175607868
; 187051827
; 212342872
; 230031014
; 241144181
; 243170643
; 252370558
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09DHY
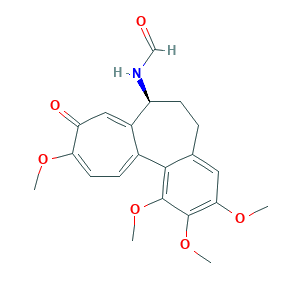
- Formula
- C21H23NO6
- Canonical SMILES
- COC1=CC=C2C(=CC1=O)C(CCC3=CC(=C(C(=C32)OC)OC)OC)NC=O
- InChI
- 1S/C21H23NO6/c1-25-17-8-6-13-14(10-16(17)24)15(22-11-23)7-5-12-9-18(26-2)20(27-3)21(28-4)19(12)13/h6,8-11,15H,5,7H2,1-4H3,(H,22,23)/t15-/m0/s1
- InChIKey
- HDSXDWASQCHADG-HNNXBMFYSA-N
|