| General Information of Drug (ID:
DR0556) |
| Drug Name |
Dydrogesterone
|
| Synonyms |
Didrogesterone; Didrogesterone [DCIT]; Diphaston; Dufaston; Duphaston; Duvaron; Dydrogesterona; Dydrogesterona [INN-Spanish]; Dydrogesteronum; Dydrogesteronum [INN-Latin]; Gestatron; Gynorest; Hydrogesterone; Hydrogestrone; Isopregnenone; Prodel; Retro-6-dehydroprogesterone; Retrone; Retroprogesterone, 6-dehydro-; Terolut; delta(6)-Retroprogesterone; delta(sup 6)-Retroprogesterone; dydrogesterone; 10alpha-Isopregnenone; 152-62-5; 6-Dehydro-retro-progesterone; 9beta,10alpha-Pregna-4,6-diene-3,20-dione; UNII-90I02KLE8K
|
| Indication |
Menorrhagia
[ICD11: GA20]
|
Approved
|
[1]
|
| Structure |
|
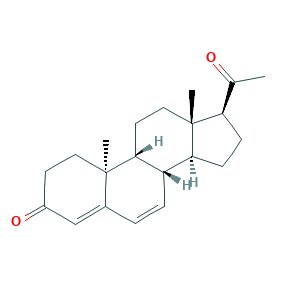 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
312.4 |
Topological Polar Surface Area |
34.1 |
| Heavy Atom Count |
23 |
Rotatable Bond Count |
1 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 9051
- PubChem SID
-
398384
; 7848280
; 7979141
; 8156439
; 11466699
; 11467819
; 11486418
; 14898944
; 15444771
; 29227669
; 46506195
; 47365375
; 47736654
; 47736655
; 48259427
; 48415933
; 49699132
; 50019401
; 50124160
; 50751954
; 53787025
; 56394852
; 56463092
; 57325399
; 71822875
; 85788500
; 87325136
; 92125727
; 103770836
; 103914481
; 104319423
; 121363398
; 124799336
; 126653040
; 126667084
; 128271591
; 134338445
; 134973758
; 135650214
; 137005553
; 139642162
; 144203980
; 152034951
; 160963725
; 162177763
; 164178107
; 170465104
; 175611963
; 179149345
; 184546093
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0F1UL
- Formula
- C21H28O2
- Canonical SMILES
- CC(=O)C1CCC2C1(CCC3C2C=CC4=CC(=O)CCC34C)C
- InChI
- 1S/C21H28O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4-5,12,16-19H,6-11H2,1-3H3/t16-,17+,18-,19+,20+,21+/m0/s1
- InChIKey
- JGMOKGBVKVMRFX-HQZYFCCVSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


