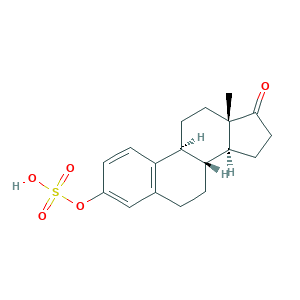
| Synonyms |
Conestoral; Estrone 3-sulfate; Estrone hydrogen sulfate; Estrone sulfate; Estrone sulphate; Par Estro; Premarin; QTL48N278K; estrone-3-sulfate; estrone-3-sulphate; 17-oxoestra-1,3,5(10)-trien-3-yl hydrogen sulfate; 3-Hydroxyestra-1,3,5(10)-trien-17-one hydrogen sulphate; 481-97-0; CHEBI:17474; Estra-1(10),2,4-trien-17-one, 3-(sulfooxy)-; UNII-QTL48N278K; [(8R,9S,13S,14S)-13-methyl-17-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-yl] hydrogen sulfate; evex
|
| Cross-matching ID |
- PubChem CID
- 3001028
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04AXP
- Formula
- C18H22O5S
- Canonical SMILES
- CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)OS(=O)(=O)O
- InChI
- 1S/C18H22O5S/c1-18-9-8-14-13-5-3-12(23-24(20,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)19/h3,5,10,14-16H,2,4,6-9H2,1H3,(H,20,21,22)/t14-,15-,16+,18+/m1/s1
- InChIKey
- JKKFKPJIXZFSSB-CBZIJGRNSA-N
|