| General Information of Drug (ID:
DR0757) |
| Drug Name |
Furosemide
|
| Synonyms |
Frusemid; Frusemide; Frusemin; Frusenex; Frusetic; Frusid; Fulsix; Fuluvamide; Furanthril; Furanthryl; Furantril; Furanturil; Furesis; Furosedon; Furosemid; Fursemid; Fursemide; Hydro-rapid; Impugan; Katlex; Lasilix; Lowpstron; Aisemide; Aluzine; Apo-Frusemide; Beronald; Desdemin; Diural; Dryptal; Errolon; Eutensin; Macasirool; Prefemin; Profemin; Promedes; Radonna; Rosemide; Rusyde; Seguril; Selectofur; Spirofur; Synephron; Transit; Trofurit; Uremide; Uresix; Urosemide; Yidoli; furosemide; 54-31-9; Disal; Fusid; Lasex; Lasix; Laxur; Lazix; Salix; Urex; Urian
|
| Indication |
Congestive heart failure
[ICD11: BD10]
|
Approved
|
[1]
|
| Structure |
|
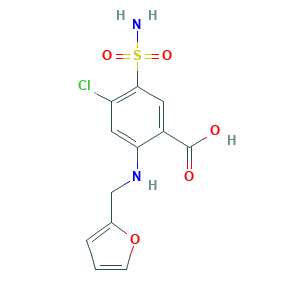 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
330.74 |
Topological Polar Surface Area |
131 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 3440
- PubChem SID
-
9229
; 140841
; 597353
; 5651429
; 7847397
; 8149353
; 8152188
; 10321703
; 11112125
; 11335750
; 11360989
; 11363994
; 11366556
; 11369118
; 11371374
; 11374398
; 11377280
; 11461961
; 11466369
; 11467489
; 11484865
; 11486058
; 11488821
; 11490253
; 11492375
; 11494914
; 11531975
; 11534249
; 12014152
; 14875224
; 17389872
; 22391430
; 24277714
; 26611753
; 26680261
; 26747275
; 26747276
; 26752834
; 26752835
; 29214787
; 29222574
; 46487913
; 46506779
; 47193739
; 47216755
; 47440227
; 47515298
; 47810733
; 47885389
; 48035085
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0PQ3G
- Formula
- C12H11ClN2O5S
- Canonical SMILES
- C1=COC(=C1)CNC2=CC(=C(C=C2C(=O)O)S(=O)(=O)N)Cl
- InChI
- 1S/C12H11ClN2O5S/c13-9-5-10(15-6-7-2-1-3-20-7)8(12(16)17)4-11(9)21(14,18)19/h1-5,15H,6H2,(H,16,17)(H2,14,18,19)
- InChIKey
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


