| General Information of Drug (ID:
DR0895) |
| Drug Name |
Itraconazole
|
| Synonyms |
Itraconazol; Itraconazol [Spanish]; Itraconazolum [Latin]; Itralek; Itrizole; Oriconazole; Orungal; Prokanazol; R-51211; SUBA Itraconazole; Sempera; Spherazole; Spherazole CR; Spherazole IR; Sporal; Sporamelt; Sporanox; Sporonox; Traconal; Triasporin; Canadiol; Candistat; Canditral; Hyphanox; cis-Itraconazole; itraconazole; (+-)-1-sec-Butyl-4-(p-(4-(p-(((2R*,4S*)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)-1-piperazinyl)phenyl)-delta(sup 2)-1,2,4-triazolin-5-one; BRN 6042047; Itrac
|
| Indication |
Blastomycosis
[ICD11: 1F22]
|
Approved
|
[1]
|
| Structure |
|
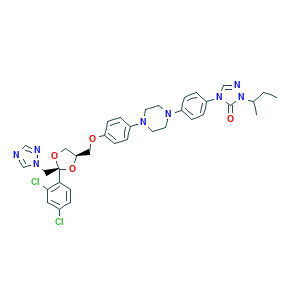 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
705.6 |
Topological Polar Surface Area |
101 |
| Heavy Atom Count |
49 |
Rotatable Bond Count |
11 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
9 |
| Cross-matching ID |
- PubChem CID
- 6917738
- PubChem SID
-
12012955
; 14839906
; 23950781
; 43529166
; 57371668
; 80687815
; 114786875
; 129477277
; 138818153
; 143350865
; 226641989
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0V4IB
- Formula
- C35H38Cl2N8O4
- Canonical SMILES
- CCC(C)N1C(=O)N(C=N1)C2=CC=C(C=C2)N3CCN(CC3)C4=CC=C(C=C4)OCC5COC(O5)(CN6C=NC=N6)C7=C(C=C(C=C7)Cl)Cl
- InChI
- 1S/C35H38Cl2N8O4/c1-3-25(2)45-34(46)44(24-40-45)29-7-5-27(6-8-29)41-14-16-42(17-15-41)28-9-11-30(12-10-28)47-19-31-20-48-35(49-31,21-43-23-38-22-39-43)32-13-4-26(36)18-33(32)37/h4-13,18,22-25,31H,3,14-17,19-21H2,1-2H3/t25?,31-,35-/m1/s1
- InChIKey
- VHVPQPYKVGDNFY-AVQIMAJZSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


