| General Information of Drug (ID:
DR0936) |
| Drug Name |
Levocarnitine
|
| Synonyms |
L-Carnitine inner salt; L-carnitine; L-carnitine Base; Lefcar; Levocarnitina; Levocarnitina [Spanish]; Levocarnitine; Levocarnitine [USAN:INN]; Levocarnitinum; Levocarnitinum [Latin]; Metina; bicarnesine; vitamin BT; (-)-Carnitine; (-)-L-Carnitine; Carniking; Carniking 50; Carnilean; Carnitene; Carnitine; Carnitine, (-)-; Carnitolo; Carnitor; Carnovis; Carrier; Karnitin; L(-)-Carnitine; L-(-)-Carnitine; (3R)-3-hydroxy-4-(trimethylammonio)butanoate; (R)-Carnitine; 541-15-1; gamma-Trimethyl-beta-hydroxybutyrobetaine
|
| Indication |
Cognitive impairment
[ICD11: 6D71]
|
Approved
|
[1]
|
| Structure |
|
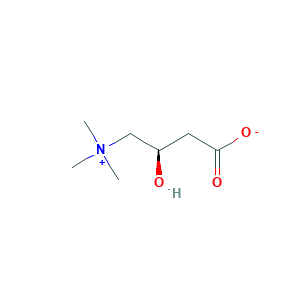 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
161.2 |
Topological Polar Surface Area |
60.4 |
| Heavy Atom Count |
11 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 10917
- PubChem SID
-
2207
; 3612
; 841764
; 7849237
; 8145671
; 8157930
; 12125266
; 15120307
; 15171033
; 24892238
; 29279278
; 46475651
; 46505864
; 48416167
; 49747058
; 49833446
; 49867038
; 53788226
; 56312758
; 56320717
; 56320718
; 57326565
; 77300350
; 85165027
; 87565251
; 91146345
; 92308023
; 93166761
; 103332887
; 103944816
; 104324626
; 115354359
; 118307976
; 124360711
; 124757780
; 124811873
; 125164584
; 126603600
; 126608844
; 131318133
; 131549872
; 134224069
; 134338232
; 134975930
; 135692459
; 136903840
; 137003561
; 141954380
; 143433319
; 143857297
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0G8SQ
- Formula
- C7H15NO3
- Canonical SMILES
- C[N+](C)(C)CC(CC(=O)[O-])O
- InChI
- 1S/C7H15NO3/c1-8(2,3)5-6(9)4-7(10)11/h6,9H,4-5H2,1-3H3/t6-/m1/s1
- InChIKey
- PHIQHXFUZVPYII-ZCFIWIBFSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


