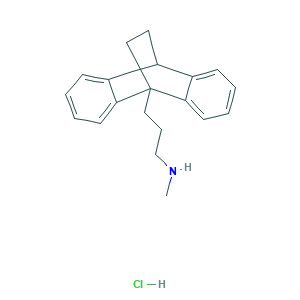
| Synonyms |
Maprotilina; Maprotilina [INN-Spanish]; Maprotilinum; Maprotilinum [INN-Latin]; Maprotylina; Maprotylina [Polish]; maprotiline; 10262-69-8; 276-Ba; 2U1W68TROF; 3-(9,10-Dihydro-9,10-ethanoanthracen-9-yl)-N-methylpropan-1-amine; 3-(9,10-Dihydro-9,10-ethanoanthracen-9-yl)propylmethylamine; 9,10-Ethanoanthracene-9(10H)-propanamine, N-methyl-; 9,10-Ethanoanthracene-9(10H)-propylamine, N-methyl-; BA-34276; BRN 2385493; CHEBI:6690; EINECS 233-599-4; N-Methyl-9,10-ethanoanthracene-9(10H)-propylamine; UNII-2U1W68TROF; Ciba 34276 Ba; Deprilept; Ludiomil; Maprotiline (hydrochloride); Maprotiline Hcl; Maprotiline hydrochloride; Maprotilline HCl; Psymion; 10347-81-6; 7C8J54PVFI; 9,10-Ethanoanthracene-9(10H)-propanamine, N-methyl-, hydrochloride; 9-(gamma-Methylaminopropyl)-9,10-dihydro-9,10-ethanoanthracene hydrochloride; BA 34276; CPD000148117; EINECS 233-758-8; MFCD00079464; MLS000069552; MLS000557000; N-Methyl-9,10-ethanoanthracene-9(10H)-propanamine hydrochloride; SMR000058834; SMR000148117; UNII-7C8J54PVFI
|
| Cross-matching ID |
- PubChem CID
- 71478
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03KQF
- Formula
- C20H24ClN
- Canonical SMILES
- CNCCCC12CCC(C3=CC=CC=C31)C4=CC=CC=C24.Cl
- InChI
- 1S/C20H23N.ClH/c1-21-14-6-12-20-13-11-15(16-7-2-4-9-18(16)20)17-8-3-5-10-19(17)20;/h2-5,7-10,15,21H,6,11-14H2,1H3;1H
- InChIKey
- NZDMFGKECODQRY-UHFFFAOYSA-N
|