| General Information of Drug (ID:
DR1068) |
| Drug Name |
Metoclopramide hydrochloride
|
| Synonyms |
Metoclopramide Intensol; Metoclopramide hydrochloride; Metoclopramide monohydrochloride; Metaclopramide; Metaclopromide; Methochlopramide; Methoclopramide; Metochlopramide; Metoclol; Metoclopramida; Metoclopramida [INN-Spanish]; Metoclopramidum; Metoclopramidum [INN-Latin]; Metocobil; Metramid; Moriperan; Parmid; Plasil; Plasil (pharmaceutical); Primperan; Reglan; Reliveran; Terperan; metoclopramide; 2-Methoxy-5-chloroprocainamide; 364-62-5; 4-Amino-5-chloro-N-(2-(diethylamino)ethyl)-2-methoxybenzamide; 5-Chloro-2-methoxyprocainamide; Metozolv; Paspertin; Primperan (tablet); Rimetin; 4-Amino-5-chloro-N-(2-(diethylamino)ethyl)-2-methoxybenzamide hydrochloride; 4-Amino-5-chloro-N-(2-(diethylamino)ethyl)-o-anisamide hydrochloride; 7232-21-5; 7B1QZY5SWZ; AHR-3070-C; C14H22ClN3O2.HCl; CCRIS 7142; EINECS 230-634-5; MFCD00058011; MLS000069667; Metoclopramide hydrochloride anhydrous; NSC 354467; SMR000058471; UNII-7B1QZY5SWZ; Clopra; DEL 1267; Elieten; Gastromax; Maxeran; METOCLOPRAMIDE HCL
|
| Indication |
Functional nausea/vomiting
[ICD11: DD90]
|
Approved
|
[1]
|
| Structure |
|
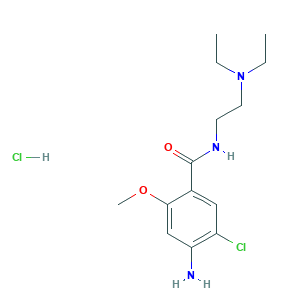 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
336.3 |
Topological Polar Surface Area |
67.6 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 23659
- CAS Number
-
- TTD Drug ID
- D08VYV
- Formula
- C14H23Cl2N3O2
- Canonical SMILES
- CCN(CC)CCNC(=O)C1=CC(=C(C=C1OC)N)Cl.Cl
- InChI
- 1S/C14H22ClN3O2.ClH/c1-4-18(5-2)7-6-17-14(19)10-8-11(15)12(16)9-13(10)20-3;/h8-9H,4-7,16H2,1-3H3,(H,17,19);1H
- InChIKey
- RVFUNJWWXKCWNS-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


