| General Information of Drug (ID:
DR1136) |
| Drug Name |
Nelarabine
|
| Synonyms |
Nelarabine; Nelarabine (Arranon); Nelzarabine; Nelzarabine (USAN); Arranon; ArranonG; Atriance; (2R,3S,4S,5R)-2-(2-Amino-6-methoxy-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol; (2R,3S,4S,5R)-2-(2-amino-6-methoxy-9H-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol; 121032-29-9; 2-Amino-9-beta-D-arabinofuranosyl-6-methoxy-9H-purine; 506U; 506U78; 60158CV180; CHEBI:63612; DSSTox_CID_26842; DSSTox_GSID_46842; DSSTox_RID_81952; GW 506U78; GW-506U78; MAY; NCGC00181098-01; NSC-686673; S1213; UNII-60158CV180
|
| Indication |
Acute lymphoblastic leukemia
[ICD11: 2B33]
|
Approved
|
[1]
|
| Structure |
|
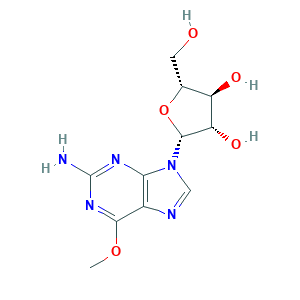 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
297.27 |
Topological Polar Surface Area |
149 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
9 |
| Cross-matching ID |
- PubChem CID
- 3011155
- PubChem SID
-
3723610
; 10039476
; 12015013
; 14775959
; 14922755
; 36059494
; 46506325
; 47206859
; 50125808
; 57410212
; 71821532
; 80796537
; 99437030
; 103771095
; 111631431
; 124757087
; 125163891
; 126592934
; 128235087
; 131296361
; 134223564
; 134338255
; 135108408
; 135611106
; 136368093
; 136375852
; 136949086
; 137006687
; 140519970
; 144074932
; 144115623
; 144206267
; 151991744
; 152059610
; 152213835
; 152344115
; 160964602
; 162011505
; 164175279
; 170464759
; 172914301
; 174477095
; 175266277
; 176484949
; 178103668
; 179116965
; 185998139
; 196376328
; 210274937
; 210280574
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0B8UJ
- Formula
- C11H15N5O5
- Canonical SMILES
- COC1=NC(=NC2=C1N=CN2C3C(C(C(O3)CO)O)O)N
- InChI
- 1S/C11H15N5O5/c1-20-9-5-8(14-11(12)15-9)16(3-13-5)10-7(19)6(18)4(2-17)21-10/h3-4,6-7,10,17-19H,2H2,1H3,(H2,12,14,15)/t4-,6-,7+,10-/m1/s1
- InChIKey
- IXOXBSCIXZEQEQ-UHTZMRCNSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


