Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1260) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
WSM-3978G
|
|||||
| Synonyms |
Perhexilene; Perhexilina; Perhexilina [INN-Spanish]; Perhexiline (INN); Perhexiline [INN:BAN]; Perhexilinum; Perhexilinum [INN-Latin]; perhexilline; (+)-2-(2,2-Dicyclohexylethyl)piperidine; (-)-2-(2,2-Dicyclohexylethyl)piperidine; 2-(2,2-Dicyclohexylethyl)piperidine; 2-[2,2-DICYCLOHEXYLETHYL]PIPERIDINE MALEATE SALT; 39648-47-0; 39648-48-1; 6621-47-2; CHEBI:35553; CHEMBL75880; EINECS 229-569-5; EINECS 252-426-3; CYXKNKQEMFBLER-UHFFFAOYSA-N; PERHEXILINE; EINECS 254-558-7; EINECS 254-559-2; Piperidine, 2-(2,2-dicyclohexylethyl)-
|
|||||
| Indication | Hypertrophic cardiomyopathy [ICD11: BC43] | Phase 3 | [1] | |||
| Structure |
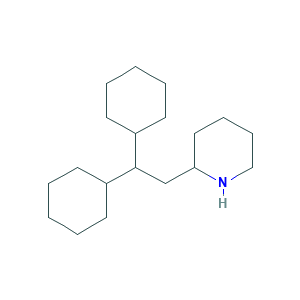 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 277.5 | Topological Polar Surface Area | 12 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 1 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

