| General Information of Drug (ID:
DR1353) |
| Drug Name |
Promethazine
|
| Synonyms |
Phargan; Phenargan; Phenerzine; Phensedyl; Pilothia; Pilpophen; Proazaimine; Proazamine; Procit; Promazinamide; Promergan; Promesan; Prometasin; Prometazin; Prometazina; Promethacon; Promethazin; Promethazinum; Promethegan; Promethiazine; Promezathine; Prorex; Protazine; Prothazin; Provigan; Pyrethia; Pyrethiazine; Tanidil; Thiergan; Vallergine; Antiallersin; Aprobit; Atosil; Avomine; Camergan; Dimapp; Diphergan; Diprazine; Diprozin; Fargan; Fenazil; Fenetazina; Fenetazine; Hiberna; Histargan; Iergigan; Isophenergan; Lilly 1516; promethazine; 60-87-7
|
| Indication |
Functional nausea/vomiting
[ICD11: DD90]
|
Approved
|
[1]
|
| Structure |
|
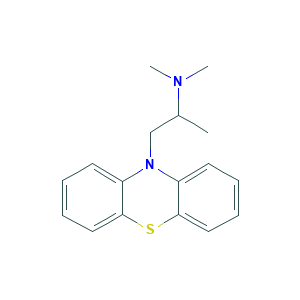 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
284.4 |
Topological Polar Surface Area |
31.8 |
| Heavy Atom Count |
20 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 4927
- PubChem SID
-
9608
; 89596
; 616468
; 5236627
; 7847560
; 7980398
; 8153034
; 10529464
; 11335180
; 11360419
; 11363762
; 11366324
; 11368886
; 11372096
; 11374835
; 11377048
; 11406271
; 11461391
; 11466916
; 11468036
; 11484535
; 11486631
; 11488656
; 11490834
; 11492963
; 11494682
; 14848899
; 24434790
; 26752304
; 29224005
; 46507798
; 47216602
; 47216603
; 47216604
; 47290955
; 47588815
; 47885231
; 48110274
; 48184815
; 48259042
; 48416477
; 49698886
; 49854482
; 50105224
; 56313067
; 56313700
; 56459433
; 57322528
; 75806049
; 85088938
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0T2XU
- Formula
- C17H20N2S
- Canonical SMILES
- CC(CN1C2=CC=CC=C2SC3=CC=CC=C31)N(C)C
- InChI
- 1S/C17H20N2S/c1-13(18(2)3)12-19-14-8-4-6-10-16(14)20-17-11-7-5-9-15(17)19/h4-11,13H,12H2,1-3H3
- InChIKey
- PWWVAXIEGOYWEE-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


