| General Information of Drug (ID:
DR1379) |
| Drug Name |
Quinidine
|
| Synonyms |
Quinact; Quinaglute; Quinalan; Quinatime; Quindine; Quinicardine; Quinidex; Quinidine sulfate; Quiniduran; Quinora; beta-Quinine; Cardioquin; Chinidin; Chinidine; Cin-Quin; Cinchonan-9-ol, 6'-methoxy-, (9S)-; Conchinin; Conchinine; Conquinine; Duraquin; ITX08688JL; Kinidin; Pitayine; chinidinum; quinidina; quinidine; (+)-Quinidine; (8R,9S)-Quinidine; (9S)-6'-Methoxycinchonan-9-ol; (S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl](6-methoxyquinolin-4-yl)methanol; 56-54-2; CHEBI:28593; CHEMBL1294; MFCD00135581; UNII-ITX08688JL
|
| Indication |
Ventricular tachyarrhythmia
[ICD11: BC71]
|
Approved
|
[1]
|
| Structure |
|
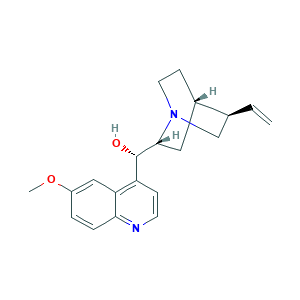 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
324.4 |
Topological Polar Surface Area |
45.6 |
| Heavy Atom Count |
24 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 441074
- ChEBI ID
-
- CAS Number
-
- Formula
- C20H24N2O2
- Canonical SMILES
- COC1=CC2=C(C=CN=C2C=C1)C(C3CC4CCN3CC4C=C)O
- InChI
- 1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1
- InChIKey
- LOUPRKONTZGTKE-LHHVKLHASA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


