| Synonyms |
Salmetedur; Salmeterol (xinafoate); Salmeterol 1-hydroxy-2-naphthoate; Salmeterol xinafonate; Serevent Inhaler and Disks; Serevent diskus; Salmeterol; Salmeterolum; Salmeterolum [Latin]; Serevent; (+-)-4-Hydroxy-alpha'-(((6-(4-phenylbutoxy)hexyl)amino)methyl)-m-xylene-alpha,alpha'-diol; (+-)-4-Hydroxy-alpha1-(((6-(4-phenylbutoxy)hexyl)amino)methyl)-1,3-benzenedimethanol; 136112-01-1; 2-(hydroxymethyl)-4-(1-hydroxy-2-{[6-(4-phenylbutoxy)hexyl]amino}ethyl)phenol; 2-(hydroxymethyl)-4-[1-hydroxy-2-[6-(4-phenylbutoxy)hexylamino]ethyl]phenol; 89365-50-4; Aeromax; Astmerole; CHEBI:64064; CPD000466295; GIIZNNXWQWCKIB-UHFFFAOYSA-N; GR 33343X; HSDB 7315; SALMATEROL; Ultrabeta; salmeterol xinafoate; 4-(1-Hydroxy-2-((6-(4-phenylbutoxy)hexyl)amino)ethyl)-2-(hydroxymethyl)phenol 1-hydroxy-2-naphthoate; 94749-08-3; Arial; Asmerole; Beglan; Betamican; DSSTox_CID_25798; DSSTox_GSID_45798; DSSTox_RID_81137; Dilamax; GR 33343 G; GR 33343X xinafoate; Inaspir; MFCD00897708; NCGC00094372-03
|
| Cross-matching ID |
- PubChem CID
- 56801
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0L5YV
- Formula
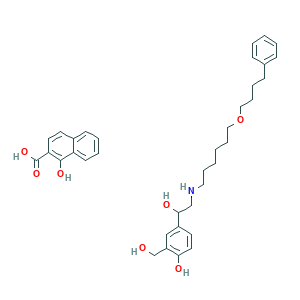
- C36H45NO7
- Canonical SMILES
- C1=CC=C(C=C1)CCCCOCCCCCCNCC(C2=CC(=C(C=C2)O)CO)O.C1=CC=C2C(=C1)C=CC(=C2O)C(=O)O
- InChI
- 1S/C25H37NO4.C11H8O3/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21;12-10-8-4-2-1-3-7(8)5-6-9(10)11(13)14/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2;1-6,12H,(H,13,14)
- InChIKey
- XTZNCVSCVHTPAI-UHFFFAOYSA-N
|