| General Information of Drug (ID:
DR1653) |
| Drug Name |
Tropisetron
|
| Synonyms |
Tropisetron (INN); Tropisetronum; Tropisetronum [INN-Latin]; Tropisteron; Lopac-T-104; Navoban (TN); Novaban; beta-Tropisetron; tropisetron; (3-endo)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl 1H-indole-3-carboxylate; 1alphaH,5alphaH-Tropan-3alpha-yl indole-3-carboxylate; 6I819NIK1W; 89565-68-4; AC1LCVDG; CHEBI:32269; CHEMBL56564; DSSTox_CID_24137; DSSTox_GSID_44137; DSSTox_RID_80108; ICF 205-930; ICS 205930; ICS-205-930; ICS-205930; TKT; UNII-6I819NIK1W; [(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] 1H-indole-3-carboxylate
|
| Indication |
Anaesthesia
[ICD11: 8E22]
|
Phase 4
|
[1]
|
| Structure |
|
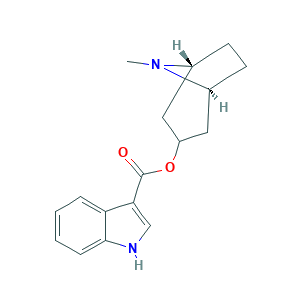 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
284.35 |
Topological Polar Surface Area |
45.3 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
3 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 656665
- PubChem SID
-
586259
; 7849191
; 7980847
; 9268089
; 11111839
; 12013648
; 15222410
; 26755041
; 32961435
; 34873056
; 47805530
; 48404460
; 57288867
; 57408379
; 78731080
; 85176985
; 92309072
; 104003611
; 104234183
; 113528005
; 124881513
; 135651284
; 136037819
; 136212975
; 137248058
; 140119381
; 140836794
; 142488962
; 144203823
; 152109096
; 162177588
; 163621151
; 163686496
; 163719193
; 170466432
; 176484497
; 179316852
; 184545081
; 184654810
; 184812293
; 198945704
; 223441795
; 226408320
; 226408321
; 226408322
; 229923840
; 238285228
; 242115154
; 245129894
; 245216191
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0K0KH
- Formula
- C17H20N2O2
- Canonical SMILES
- CN1C2CCC1CC(C2)OC(=O)C3=CNC4=CC=CC=C43
- InChI
- 1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3/t11-,12+,13+
- InChIKey
- ZNRGQMMCGHDTEI-FUNVUKJBSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


