| General Information of Drug (ID:
DR1878) |
| Drug Name |
LY-2484595
|
| Synonyms |
Evacetrapib; Evacetrapib (LY2484595); Evacetrapib (USAN); Evacetrapib [USAN:INN]; IHIUGIVXARLYHP-YBXDKENTSA-N; LY 2484595; LY-2484595; LY2484595; SCHEMBL108367; SCHEMBL108602; SCHEMBL141340; Tube726; evacetrapib-ly2484595; (1S,4r)-4-(((S)-5-((3,5-bis(trifluoromethyl)benzyl)(2-methyl-2H-tetrazol-5-yl)amino)-7,9-dimethyl-2,3,4,5-tetrahydro-1H-benzo[b]azepin-1-yl)methyl)cyclohexanecarboxylic acid; 1186486-62-3; 51XWV9K850; AK172427; C31H36F6N6O2; CHEMBL2017179; EX-A621; GTPL8401; HMS3651J12; KS-000007CQ; UNII-51XWV9K850
|
| Indication |
Myocardial infarction
[ICD11: BA41]
|
Phase 3
|
[1]
|
| Structure |
|
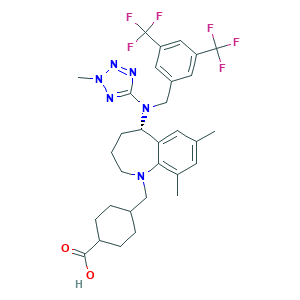 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
638.6 |
Topological Polar Surface Area |
87.4 |
| Heavy Atom Count |
45 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
13 |
| Cross-matching ID |
- PubChem CID
- 49836058
- PubChem SID
-
103911733
; 135268436
; 135626839
; 137240261
; 141386796
; 141386797
; 152258542
; 160647376
; 160699241
; 160860498
; 161005253
; 162221695
; 164194109
; 170504191
; 172096382
; 198940733
; 223390433
; 223471431
; 224462115
; 225848218
; 226482340
; 226482535
; 226508337
; 244317218
; 248331392
; 249734743
; 252155357
; 252160892
; 252166611
; 252215734
; 252471646
; 252810963
- CAS Number
-
- TTD Drug ID
- D02XXN
- Formula
- C31H36F6N6O2
- Canonical SMILES
- CC1=CC(=C2C(=C1)C(CCCN2CC3CCC(CC3)C(=O)O)N(CC4=CC(=CC(=C4)C(F)(F)F)C(F)(F)F)C5=NN(N=N5)C)C
- InChI
- 1S/C31H36F6N6O2/c1-18-11-19(2)27-25(12-18)26(5-4-10-42(27)16-20-6-8-22(9-7-20)28(44)45)43(29-38-40-41(3)39-29)17-21-13-23(30(32,33)34)15-24(14-21)31(35,36)37/h11-15,20,22,26H,4-10,16-17H2,1-3H3,(H,44,45)/t20?,22?,26-/m0/s1
- InChIKey
- IHIUGIVXARLYHP-UXNJHFGPSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


