| General Information of Drug (ID:
DR1886) |
| Drug Name |
AZD-2171
|
| Synonyms |
Cediranib; Cediranib (AZD217); Cediranib (AZD2171); Cedirannib; NQU9IPY4K9; Recentin; ZD-2171; 288383-20-0; 4-((4-Fluoro-2-methyl-1H-indol-5-yl)oxy)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy)quinazoline; 4-(4-Fluoro-2-methylindol-5-yloxy)-6-methoxy-7-[3-(pyrrolidin-1-yl)propoxy]quinazoline; 4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-(3-pyrrolidin-1-ylpropoxy)quinazoline; AZD 2171; AZD-2171; AZD2171; CHEMBL491473; NSC-732208; UNII-NQU9IPY4K9
|
| Indication |
Lung cancer
[ICD11: 2C25]
|
Phase 3
|
[1]
|
| Structure |
|
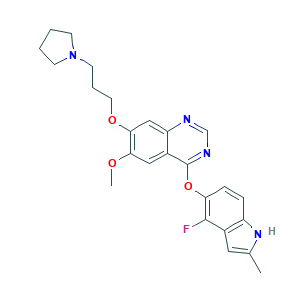 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
450.5 |
Topological Polar Surface Area |
72.5 |
| Heavy Atom Count |
33 |
Rotatable Bond Count |
8 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 9933475
- PubChem SID
-
14906490
; 24208254
; 26746631
; 57373832
; 75977042
; 96025564
; 96079499
; 99432367
; 103592924
; 103905340
; 109692967
; 123110211
; 124756936
; 124766796
; 125163743
; 126666991
; 126730931
; 129754723
; 131465106
; 134221888
; 134339006
; 134964400
; 135194712
; 135697662
; 135727476
; 136368003
; 136920391
; 137006068
; 137275941
; 139208216
; 144115802
; 152258121
; 152344142
; 160646960
; 162011780
; 162037386
; 162200059
; 163884606
; 164041832
; 174528685
; 175426860
; 177748726
; 178102292
; 180190844
; 185998790
; 187071991
; 198958766
; 198993121
; 203355740
; 223384883
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09VMI
- Formula
- C25H27FN4O3
- Canonical SMILES
- CC1=CC2=C(N1)C=CC(=C2F)OC3=NC=NC4=CC(=C(C=C43)OC)OCCCN5CCCC5
- InChI
- 1S/C25H27FN4O3/c1-16-12-17-19(29-16)6-7-21(24(17)26)33-25-18-13-22(31-2)23(14-20(18)27-15-28-25)32-11-5-10-30-8-3-4-9-30/h6-7,12-15,29H,3-5,8-11H2,1-2H3
- InChIKey
- XXJWYDDUDKYVKI-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


