Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR2435) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Isoflavone
|
|||||
| Synonyms |
Isoflavone; Isoflavone (8CI); Isoflavone skeleton; LS-191186; MCULE-2586547916; OVO2KUW8H8; SBB068618; SCHEMBL8028; ST098957; VN10014; ZINC895390; isoflavon; 3-Phenylchromone; 3-phenyl-4H-1-benzopyran-4-one; 3-phenyl-4H-chromen-4-one; 3-phenylchromen-4-one; 4H-1-Benzopyran-4-one, 3-phenyl-; 574-12-9; AC-12802; AK114020; AKOS015918505; AX8135136; BCP22856; BCP9000133; CCG-214095; CHEBI:18220; CHEMBL366460; CS-W006405; DB12007; DS-6374; DTXSID90205986; KS-00000GFW; NSC 135405; NSC-135405; NSC135405; GOMNOOKGLZYEJT-UHFFFAOYSA-N; UNII-OVO2KUW8H8
|
|||||
| Indication | Asthma [ICD11: CA23] | Phase 4 | [1] | |||
| Structure |
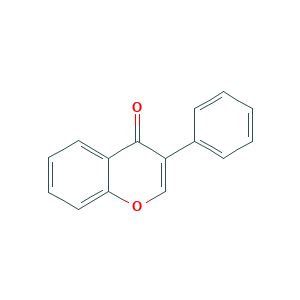 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 222.24 | Topological Polar Surface Area | 26.3 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 2 | |||
| Cross-matching ID | ||||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

