| General Information of Drug (ID:
DR2500) |
| Drug Name |
Fenofibric acid
|
| Synonyms |
Fenofibric acid; Procetofenic acid; 2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropanoic acid; 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoic acid; NSC 281318; Trilipix; alpha 1081; LF 178 acid; UNII-BGF9MN2HU1; LF 153; C17H15ClO4; CCRIS 7302; EINECS 255-626-9; BGF9MN2HU1; CHEMBL981; BRN 2058973; CHEBI:83469; NSC-281318; 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic Acid; ABT-335; AK117112; Propanoic acid, 2-(4-(4-chlorobenzoyl)phenoxy)-2-methyl-; 2-{4-[(4-chlorophenyl)carbonyl]phenoxy}-2-methylpropanoic acid; FNF Acid; W-106287; 2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropionic acid; 2-[4-(4-Chloro-benzoyl)-phenoxy]-2-methyl-propionic acid (Fenofibric acid); ABT 335; Propanoic acid, 2-[4-(4-chlorobenzoyl)phenoxy]-2-methyl-; feno-fibric acid; Fibricor (TN); Fenofibrate free acid; FENOFIBRIC ACID; SCHEMBL16377; Fenofibrate related compound b; GTPL2662; ZINC1984; DTXSID8041030; BDBM28700; MQOBSOSZFYZQOK-UHFFFAOYSA-N; BCP22437; HY-B0760; KS-00000MR0; Fenofibric acid, analytical standard; LF-153; NSC281318; s4527; AKOS015889489; API0000561; 42017-89-0
|
| Indication |
Dyslipidaemia
[ICD11: 5C81]
|
Approved
|
[1]
|
| Structure |
|
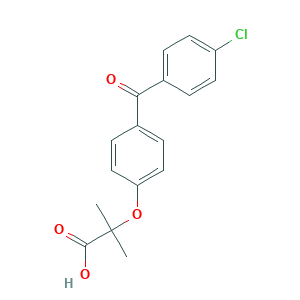 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
318.7 |
Topological Polar Surface Area |
63.6 |
| Heavy Atom Count |
22 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 64929
- PubChem SID
-
143270
; 7671261
; 8189389
; 15988368
; 29203742
; 43121787
; 50022223
; 50826157
; 56312055
; 56314036
; 58097888
; 81049613
; 85171876
; 87359585
; 103256438
; 103853918
; 104332417
; 118048388
; 118212615
; 125350317
; 126616576
; 126650397
; 126665945
; 128633517
; 134351110
; 135023245
; 135650256
; 136990671
; 137008576
; 139809304
; 143755763
; 152028908
; 162175168
; 162263030
; 163415486
; 164785582
; 172913777
; 174529345
; 175265316
; 175611044
; 184538157
; 187072366
; 198991585
; 210279847
; 210282170
; 223352414
; 223385806
; 223448217
; 223519115
; 223669964
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0NF1U
- Formula
- C17H15ClO4
- Canonical SMILES
- CC(C)(C(=O)O)OC1=CC=C(C=C1)C(=O)C2=CC=C(C=C2)Cl
- InChI
- 1S/C17H15ClO4/c1-17(2,16(20)21)22-14-9-5-12(6-10-14)15(19)11-3-7-13(18)8-4-11/h3-10H,1-2H3,(H,20,21)
- InChIKey
- MQOBSOSZFYZQOK-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


