| Synonyms |
Ramipril; Ramace; Ramipril (USP/INN); Ramipril [USAN:INN:BAN]; Ramiprilum; Ramiprilum [Latin]; Triatec; Tritace; Tritace (TN); Vesdil; Zabien; Acovil; Almirall Brand of Ramipril; Altace; Altace (TN); Astra Brand of Ramipril; AstraZeneca Brand of Ramipril; Aventis Brand of Ramipril; Aventis Pharma Brand of Ramipril; Carasel; Cardace; Hoechst Brand of Ramipril; Hypren; Hytren; Lostapres; Monarch Brand of Ramipril; Naprix; Pramace; Pramace (discontinued); Promed Brand of (2 S ,3 aS,6 aS)-1[(S)-N-[(S)-1-Carboxy-3-phenylpropyl] alanyl] octahydrocyclopenta [ b ]pyrrole-2-carboxylic acid, 1-ethyl ester; (2S,3aS,6aS)-1-((S)-2-((S)-1-ethoxy-1-oxo-4-phenylbutan-2-ylamino)propanoyl) octahydrocyclopenta[b]pyrrole-2-carboxylic acid; (2S,3aS,6aS)-1-((S)-N-((S)-1-Carboxy-3-phenylpropyl)alanyl)octahydrocyclopenta(b)pyrrole-2-carboxylic acid, 1-ethyl ester; (2S,3aS,6aS)-1-((S)-N-((S)-1-Ethoxycarbonyl-3-phenylpropyl)alanyl)octahydrocyclopenta(b)pyrrol-2-carbonsaeure; (2S,3aS,6aS)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-3,3a,4,5,6,6a-hexahydro-2H-cyclopenta[b]pyrrole-2-carboxylic acid; (2S,3aS,6aS)-1-[(2S)-2-{[(1S)-1-ethoxycarbonyl-3-phenylpropyl]amino}propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid; (2S,3aS,6aS)-1-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid (non-preferred name); (2S-(1(R*(R*)),2alpha,3abeta,6abeta))-1-(2-((1-(Ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)octahydrocyclopenta(b)pyrrole-2-carboxylic acid; (2s,3as,6as)-1((s)-n-((s)-1-carboxy-3-phenylpropyl)alanyl)octahydrocyclopenta(b)pyrrole-2-carboxylic; (2s,3as,6as)-1-((s)-2-((s)-1-ethoxy-1-oxo-4-phenylbutan-2-ylamino)propanoyl)-octahydrocyclopenta[b]p; (2s,3as,6as)-1-[(s)-2-((s)-1-ethoxycarbonyl-3-phenyl-propylamino)-propionyl]-octahydro-cyclopenta[b]; Delix; HOE 498; HOE498; Hoe-498; N-(1S-carboethoxy-3-phenylpropyl)-S-alanyl-cis,endo-2-azabicyclo[3.3.0]octane-3S-carboxylic Acid; Quark; Ramipro, Tritace, Altace, Prilace, Ramipril; [2S,3aS,6aS]-1-[(2S)-2-[[(1S)-1-(Ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid; [2s,3as,6as]-1-[(2s)-2-[[(1s)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]octahydrocyclopen
|
| Cross-matching ID |
- PubChem CID
- 5362129
- PubChem SID
-
7847487
; 7980483
; 11364914
; 11367476
; 11370038
; 11372946
; 11374996
; 11378208
; 11491609
; 11493083
; 11495772
; 11528643
; 11533329
; 12012944
; 14904601
; 14929182
; 17184929
; 24724586
; 26612834
; 26719894
; 39384402
; 46386770
; 46506390
; 47721705
; 48244488
; 48416510
; 49664947
; 49681721
; 50122729
; 53787042
; 57362088
; 76755897
; 81093204
; 85279379
; 92308080
; 92308314
; 92309227
; 92712074
; 93166961
; 103339774
; 103984211
; 104170125
; 104179208
; 104253384
; 114149719
; 117664421
; 121362522
; 124658840
; 124757502
; 124799903
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01STB
- Formula
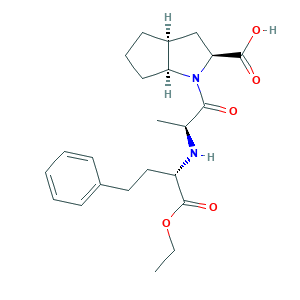
- C23H32N2O5
- Canonical SMILES
- CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2C3CCCC3CC2C(=O)O
- InChI
- 1S/C23H32N2O5/c1-3-30-23(29)18(13-12-16-8-5-4-6-9-16)24-15(2)21(26)25-19-11-7-10-17(19)14-20(25)22(27)28/h4-6,8-9,15,17-20,24H,3,7,10-14H2,1-2H3,(H,27,28)/t15-,17-,18-,19-,20-/m0/s1
- InChIKey
- HDACQVRGBOVJII-JBDAPHQKSA-N
|