| Synonyms |
6-(4-Fluorophenyl)-2,3-dihydro-5-(4-pyridinyl)imidazo(2,1-b)thiazole; skf-86002; 72873-74-6; Skf 86002; F 86002; F 86002-A(2); UNII-9R6QDF1UO7; 9R6QDF1UO7; CHEMBL313417; 5-(4-Pyridyl)-6-(4-fluorophenyl)-2,3-dihydroimidazo(2,1-b)-thiazole; 4-[6-(4-fluorophenyl)-2H,3H-imidazo[2,1-b][1,3]thiazol-5-yl]pyridine; 6-(4-fluorophenyl)-5-(4-pyridyl)-2,3-dihydroimidazo[2,1-b]thiazole; 6-(4-Fluorophenyl)-2,3-dihydro-5-(4-pyridyl)imidazo[2,1-b]thiazole; Imidazo(2,1-b)thiazole,; SK&F 86002
|
| Cross-matching ID |
- PubChem CID
- 49787172
- TTD Drug ID
- D05VVX
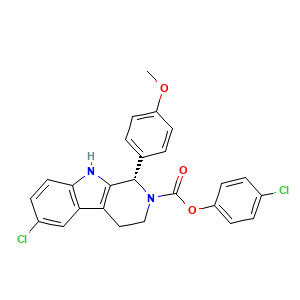
- Formula
- C25H20Cl2N2O3
- Canonical SMILES
- COC1=CC=C(C=C1)[C@H]2C3=C(CCN2C(=O)OC4=CC=C(C=C4)Cl)C5=C(N3)C=CC(=C5)Cl
- InChI
- InChI=1S/C25H20Cl2N2O3/c1-31-18-7-2-15(3-8-18)24-23-20(21-14-17(27)6-11-22(21)28-23)12-13-29(24)25(30)32-19-9-4-16(26)5-10-19/h2-11,14,24,28H,12-13H2,1H3/t24-/m0/s1
- InChIKey
- SRSHBZRURUNOSM-DEOSSOPVSA-N
|