| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
|
| Cross-matching ID |
- PubChem CID
- 36314
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0C4RB
- Formula
- C47H51NO14
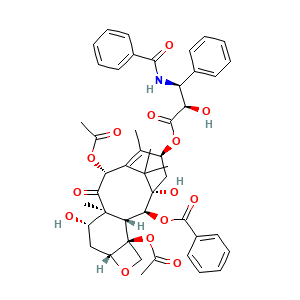
- Canonical SMILES
- CC1=C2[C@H](C(=O)[C@@]3([C@H](C[C@@H]4[C@]([C@H]3[C@@H]([C@@](C2(C)C)(C[C@@H]1OC(=O)[C@@H]([C@H](C5=CC=CC=C5)NC(=O)C6=CC=CC=C6)O)O)OC(=O)C7=CC=CC=C7)(CO4)OC(=O)C)O)C)OC(=O)C
- InChI
- InChI=1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)5/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)/t31-,32-,33+,35-,36+,37+,38-,40-,45+,46-,47+/m0/s1
- InChIKey
- RCINICONZNJXQF-MZXODVADSA-N
|