| Synonyms |
Active methionine; Ademetionine; AdoMet; S-(5'-Adenosyl)-L-methionine; S-adenosyl methionine; S-adenosyl-L-methionine; S-adenosyl-methionine; S-adenosylmethionine; SCHEMBL599664; adenosylmethionine; (S,S)-AdoMet; 2-S-adenosyl-L-methionine; 29908-03-0; 485-80-3; AC1L1S8L; Adenosine, 5'-[[(3S)-3-amino-3-carboxypropyl]methylsulfonio]-5'-deoxy-; CHEBI:15414; CHEBI:33440; CHEBI:33442; CHEMBL224120; CTK1A2085; GTPL4786; NCGC00167546-02; S-(5'-deoxyadenosin-5'-yl)-L-methionine; SAM; SAMe; SCHEMBL25177; SCHEMBL59293; bmse000059
|
| Cross-matching ID |
- PubChem CID
- 34756
- PubChem SID
-
584705
; 584819
; 585530
; 585647
; 819953
; 820416
; 822522
; 827273
; 827276
; 827431
; 827923
; 827984
; 828011
; 828013
; 828789
; 830207
; 831396
; 831453
; 831806
; 831932
; 832635
; 833168
; 834388
; 834611
; 834731
; 838314
; 838315
; 6435774
; 6435784
; 8017006
; 8018772
; 8024112
; 8028062
; 8145813
; 8147098
; 8147099
; 8173805
; 10318367
; 10318534
; 10322395
; 10322405
; 11532836
; 11538426
; 14709946
; 14716693
; 16477986
; 17404326
; 17422915
; 17422968
; 17422983
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U3YU
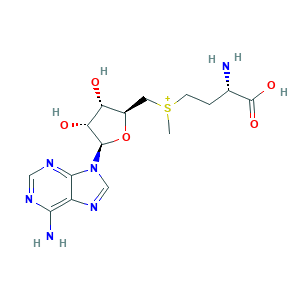
- Formula
- C15H23N6O5S+
- Canonical SMILES
- C[S+](CCC(C(=O)O)N)CC1C(C(C(O1)N2C=NC3=C(N=CN=C32)N)O)O
- InChI
- 1S/C15H22N6O5S/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/p+1/t7-,8+,10+,11+,14+,27?/m0/s1
- InChIKey
- MEFKEPWMEQBLKI-AIRLBKTGSA-O
|