| Synonyms |
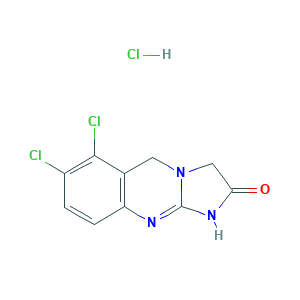
Agrelin; Agrylin; Agrylin (TN); Anagrelid hydrochlorid; Anagrelide (hydrochloride); Anagrelide HCL; Anagrelide hydrochloride [USAN]; BL 4162A; BL-4162A; BL4162A; CHEBI:55345; Thromboreductin; UNII-VNS4435G39; VNS4435G39; Xagrid; Anagrelide; Anagrelida; Anagrelidum; 6,7-Dichloro-5,10-dihydroimidazo[2,1-b]quinazolin-2(3H)-one; Anagrelide [INN:BAN]; Anagrelidum [INN-Latin]; Anagrelida [INN-Spanish]; C10H7Cl2N3O; UNII-K9X45X0051; BL 416201; HSDB 7325; CHEMBL760; BRN 0619582; 6,7-Dichloro-1,5-dihydroimidazo(2,1-b)quinazolin-2(3H)-one; 6,7-Dichlor-1,5-dihydroimidazo(2,1-b)chinazolin-2(3H)-on; CHEBI:142290; 6,7-dichloro-5,10-dihydro-3H-imidazo[2,1-b]quinazolin-2-one; K9X45X0051; 68475-42-3; 58579-51-4; 6,7-Dichloro-1,5-dihydroimidazo(2,1-b)-quinazolin-2(3H)-one monohydrochloride; 6,7-Dichloro-5,10-dihydroimidazo[2,1-b]quinazolin-2(3H)-one hydrochloride; 6,7-dichloro-1,5-dihydroimidazo[2,1-b]quinazolin-2(3H)-one hydrochloride; ANAGRELIDE HYDROCHLORIDE
|