| General Information of Drug (ID:
DR0266) |
| Drug Name |
Capecitabine
|
| Prodrug Info |
Capecitabine is the prodrug of Fluorouracil
|
| Synonyms |
Capecitibine; Capiibine; Captabin; Ro 09-1978; Ro 09-1978/000; Xeloda; 154361-50-9; 5'-Deoxy-5-fluoro-N-((pentyloxy)carbonyl)cytidine; CAPECITABINE; 5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine; Cytidine, 5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]-; N(4)-Pentyloxycarbonyl-5'-deoxy-5-fluorocytidine; Pentyl 1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinecarbamate; R340; UNII-6804DJ8Z9U
|
| Indication |
Colon cancer
[ICD11: 2B90]
|
Approved
|
[1]
|
| Structure |
|
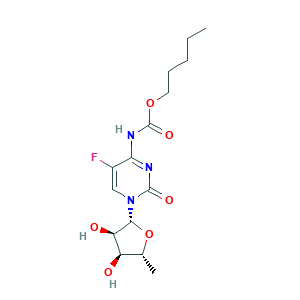 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
359.35 |
Topological Polar Surface Area |
121 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 60953
- PubChem SID
-
583040
; 7848286
; 7978485
; 8187136
; 12014895
; 14828191
; 14925873
; 29214852
; 43118267
; 46508686
; 49960125
; 53790473
; 57314195
; 71821403
; 74459733
; 99436918
; 103724218
; 104322032
; 117506114
; 117673331
; 118048469
; 119526524
; 124757042
; 125163846
; 126653765
; 126671131
; 127925204
; 134338046
; 134358377
; 135017419
; 135693779
; 135723537
; 136368008
; 136375551
; 136949108
; 137005622
; 140014282
; 143493385
; 144115785
; 144205751
; 152059549
; 152235778
; 152258963
; 160647810
; 160964435
; 162011675
; 163304897
; 164175281
; 164194987
; 165245546
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00HCQ
- Formula
- C15H22FN3O6
- Canonical SMILES
- CCCCCOC(=O)NC1=NC(=O)N(C=C1F)C2C(C(C(O2)C)O)O
- InChI
- 1S/C15H22FN3O6/c1-3-4-5-6-24-15(23)18-12-9(16)7-19(14(22)17-12)13-11(21)10(20)8(2)25-13/h7-8,10-11,13,20-21H,3-6H2,1-2H3,(H,17,18,22,23)/t8-,10-,11-,13-/m1/s1
- InChIKey
- GAGWJHPBXLXJQN-UORFTKCHSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


