| Synonyms |
Clenbuterolum; Clenbuterol; Clenbuterolum [INN-Latin]; Monores; Planipart; STJMRWALKKWQGH-UHFFFAOYSA-N; clenbuterol; (+/-)-clenbuterol; 1-(4-Amino-3,5-dichlorophenyl)-2-(tert-butylamino)ethanol; 37148-27-9; 4-Amino-3,5-dichloro-alpha-(((1,1-dimethylethyl)amino)methyl)benzenemethanol; 4-Amino-alpha-((tert-butylamino)methyl)-3,5-dichlorobenzyl alcohol; BENZENEMETHANOL, 4-AMINO-3,5-DICHLORO-alpha-(((1,1-DIMETHYLETHYL)AMINO)METHYL)-; BRN 1076467; CHEBI:174690; EINECS 253-366-0; NAB 365; NAB-365; NCGC00163150-01
|
| Cross-matching ID |
- PubChem CID
- 2783
- PubChem SID
-
4445365
; 7980738
; 8151795
; 11335969
; 11361208
; 11364458
; 11367020
; 11369582
; 11371928
; 11374205
; 11374656
; 11377744
; 11462180
; 11466373
; 11467493
; 11486066
; 11490790
; 11492558
; 11492913
; 11495378
; 15222099
; 26756514
; 29216418
; 29221938
; 46508373
; 47216807
; 47589036
; 47885449
; 47959784
; 48035160
; 48110483
; 48334530
; 48415793
; 49698946
; 49880554
; 50111385
; 50111386
; 50294329
; 51092009
; 53789319
; 56312093
; 56312823
; 56312919
; 57321457
; 57560470
; 85084908
; 85373776
; 85788859
; 85856301
; 92308851
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X5NX
- Formula
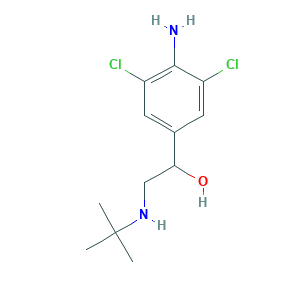
- C12H18Cl2N2O
- Canonical SMILES
- CC(C)(C)NCC(C1=CC(=C(C(=C1)Cl)N)Cl)O
- InChI
- 1S/C12H18Cl2N2O/c1-12(2,3)16-6-10(17)7-4-8(13)11(15)9(14)5-7/h4-5,10,16-17H,6,15H2,1-3H3
- InChIKey
- STJMRWALKKWQGH-UHFFFAOYSA-N
|