Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0424) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Daunorubicin
|
|||||
| Synonyms |
Daunamycin; Daunarubicinum; DaunoXome; DaunoXome (TN); Daunoblastin; Daunomycin; Daunorrubicina; Daunorubicin [INN:BAN]; Daunorubicine; Daunorubicinum; Daunorubicinum [INN-Latin]; FI6339; Leukaemomycin C; Ondena; RCRA waste no. U059; Acetyladriamycin; Anthracyline; Cerubidin; Cerubidine; RP 13057; Rubidomycin; Rubomycin; Rubomycin C; ZS7284E0ZP; daunorubicin; (+)-Daunomycin; 20830-81-3; C27H29NO10; CCRIS 914; CHEBI:41977; EINECS 244-069-7; HSDB 5095; NCI-C04693; NSC 83142; NSC-82151; UNII-ZS7284E0ZP
|
|||||
| Indication | Acute myeloid leukaemia [ICD11: 2A60] | Approved | [1] | |||
| Structure |
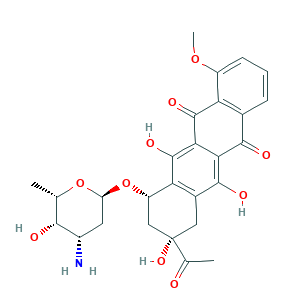 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 527.5 | Topological Polar Surface Area | 186 | ||
| Heavy Atom Count | 38 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 11 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Enzyme Kinetic Data of This Drug | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Daunorubicin was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | The effect of new lipophilic chelators on the activities of cytosolic reductases and P450 cytochromes involved in the metabolism of anthracycline antibiotics: studies in vitro. Physiol Res. 2004;53(6):683-91. | |||||
| 3 | Lack of mechanism-based inactivation of rat hepatic microsomal cytochromes P450 by doxorubicin. Can J Physiol Pharmacol. 1999 Aug;77(8):589-97. | |||||
| 4 | Flavonoids as inhibitors of human carbonyl reductase 1. Chem Biol Interact. 2008 Jul 30;174(2):98-108. | |||||
| 5 | Naturally occurring variants of human CBR3 alter anthracycline in vitro metabolism. J Pharmacol Exp Ther. 2010 Mar;332(3):755-63. | |||||
| 6 | Daunorubicin metabolites in human urine J Pharmacol Exp Ther. 1975 Oct;195(1):41-9. | |||||
| 7 | Daunorubicin and Its Active Metabolite Pharmacokinetic Profiles in Acute Myeloid Leukaemia Patients: A Pharmacokinetic Ancillary Study of the BIG-1 Trial. Pharmaceutics. 2022 Apr 5;14(4):792. doi: 10.3390/pharmaceutics14040792. | |||||
| 8 | The electromembrane extraction of pharmaceutical compounds from animal tissues. Anal Chim Acta. 2021 Sep 8;1177:338742. doi: 10.1016/j.aca.2021.338742. | |||||
| 9 | Electromembrane extraction of anthracyclines from plasma: Comparison with conventional extraction techniques. Talanta. 2021 Feb 1;223(Pt 2):121748. doi: 10.1016/j.talanta.2020.121748. | |||||
| 10 | U. S. FDA Label -Daunorubicin | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

