| Synonyms |
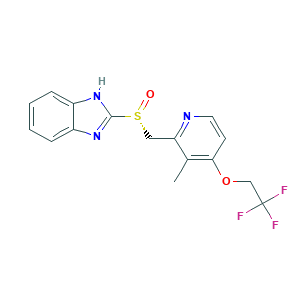
Dexilant Solutab; Dexlansoprazole (INN/USAN); Dexlansoprazole [USAN:INN]; Kapidex; R-(+)-LANSOPRAZOLE; T 168390; T-168390; TAK 390; TAK-390; TAK-390MR; UYE4T5I70X; dexilant; dexlansoprazole; (R)-2-(((3-Methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)sulfinyl)-1H-benzo[d]imidazole; (R)-Lansoprazole; 138530-94-6; 2-((R)-((3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl)methyl)sulfinyl)-1H-benzimidazole; 2-[(R)-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methylsulfinyl]-1H-benzimidazole; AK170558; UNII-UYE4T5I70X
|
| Cross-matching ID |
- PubChem CID
- 9578005
- PubChem SID
-
14901842
; 15375021
; 23998121
; 25819922
; 44715913
; 80665304
; 89736101
; 96025586
; 104179070
; 104234371
; 126682042
; 128604168
; 135156589
; 137931058
; 140767605
; 162189572
; 164178109
; 164761527
; 170503333
; 175265917
; 175269426
; 175611039
; 178102134
; 184527042
; 185965235
; 210274918
; 210280553
; 223658647
; 223798807
; 226429139
; 241033947
; 251879797
; 252151427
; 252431524
; 252811271
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06YYD
- Formula
- C16H14F3N3O2S
- Canonical SMILES
- CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3N2)OCC(F)(F)F
- InChI
- 1S/C16H14F3N3O2S/c1-10-13(20-7-6-14(10)24-9-16(17,18)19)8-25(23)15-21-11-4-2-3-5-12(11)22-15/h2-7H,8-9H2,1H3,(H,21,22)/t25-/m1/s1
- InChIKey
- MJIHNNLFOKEZEW-RUZDIDTESA-N
|