Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0470) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
CR-2017
|
|||||
| Synonyms |
Dexloxiglumida; Dexloxiglumida [INN-Spanish]; Dexloxiglumide; Dexloxiglumide [INN]; Dexloxiglumidum; Dexloxiglumidum [INN-Latin]; PB12713; PubChem16049; Q-4119; SCHEMBL366142; ZINC3801027; (R)-4-(3,4-Dichlorobenzamido)-5-((3-methoxypropyl)(pentyl)amino)-5-oxopentanoic acid; 119817-90-2; 69DY40RH9B; A11769; AC1L24A8; AC1Q3MXI; AJ-45649; AK129760; AKOS000279054; AS-35102; AX8123879; CC-26372; CHEBI:135747; CHEMBL550781; CR-2017; CS-0054881; DB04856; DTXSID50152604; FT-0603098; GTPL889; HY-128878; KS-000007QQ; MFCD00866773; UNII-69DY40RH9B
|
|||||
| Indication | Irritable bowel syndrome [ICD11: DD91] | Phase 3 | [1] | |||
| Structure |
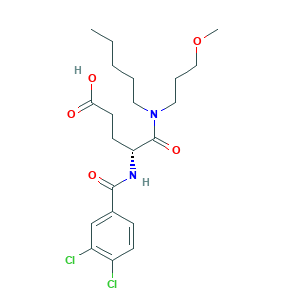 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 461.4 | Topological Polar Surface Area | 95.9 | ||
| Heavy Atom Count | 30 | Rotatable Bond Count | 14 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 5 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

