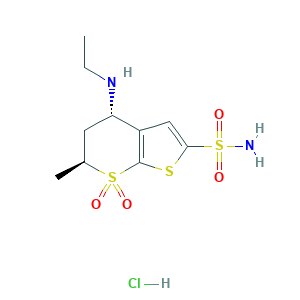
| Synonyms |
Dorzolamide (hydrochloride); Dorzolamide HCl; Dorzolomide hydrochloride; Dorzolamid; Dorzolamide; Dorzolamida; Dorzolamide (DZA); Dorzolamide (INN); Dorzolamide [INN:BAN]; Dorzolamidum; SR-05000001449; UNII-9JDX055TW1; (4S,6S)-4-(ethylamino)-6-methyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide; (4S,6S)-4-(ethylamino)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide; (4S-TRANS)-4-(ETHYLAMINO)-5,6-DIHYDRO-6-METHYL-4H-THIENO(2,3-B)THIOPYRAN-2-SULFONAMIDE-7,7-DIOXIDE; 120279-96-1; 4S,6S-Dorzolamide; 9JDX055TW1; CHEBI:4702; L 671152; L-671,152; MK 0507; MK-0507; MK-507; QZO5366EW7; Trusopt; UNII-QZO5366EW7; (4S,6S)-4-(ethylamino)-6-methyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide hydrochloride; (4S,6S)-4-(ethylamino)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide hydrochloride; 130693-82-2; CHEBI:4703; Cosopt; DORZOLAMIDE HYDROCHLORIDE; DSSTox_CID_25530; DSSTox_GSID_45530; DSSTox_RID_80933
|
| Cross-matching ID |
- PubChem CID
- 6918132
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05UYW
- Formula
- C10H17ClN2O4S3
- Canonical SMILES
- CCNC1CC(S(=O)(=O)C2=C1C=C(S2)S(=O)(=O)N)C.Cl
- InChI
- 1S/C10H16N2O4S3.ClH/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16;/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16);1H/t6-,8-;/m0./s1
- InChIKey
- OSRUSFPMRGDLAG-QMGYSKNISA-N
|