| Synonyms |
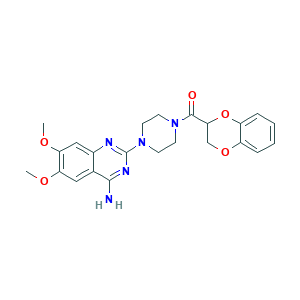
Doxazosin [INN:BAN]; Doxazosina; Doxazosina [Spanish]; Doxazosine; Doxazosine [French]; Doxazosinum; Doxazosinum [Latin]; RUZYUOTYCVRMRZ-UHFFFAOYSA-N; UK 33274; doxazosin; 1-(4-Amino-6,7-Dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl)piperazin; 1-(4-Amino-6,7-dimethoxy-2-chinazolinyl)-4-(2,3-dihydro-1,4-benzodioxixin-2-ylcarbonyl)piperazin; 2-[4-(2,3-dihydro-1,4-benzodioxin-2-ylcarbonyl)piperazin-1-yl]-6,7-dimethoxyquinazolin-4-amine; 74191-85-8; C23H25N5O5; CHEBI:4708
|
| Cross-matching ID |
- PubChem CID
- 3157
- PubChem SID
-
9184
; 4407398
; 7979127
; 8152011
; 11222167
; 11466886
; 11468006
; 11486532
; 14784191
; 24278398
; 29222299
; 46506825
; 47298998
; 47298999
; 47299000
; 47670375
; 47744452
; 47744453
; 47818609
; 48415923
; 49698667
; 50011218
; 50105246
; 50308562
; 56311128
; 56311129
; 56313874
; 56464101
; 57321640
; 85209457
; 85787347
; 90341261
; 90342475
; 92241035
; 92309056
; 92710466
; 96024570
; 103194429
; 103930616
; 104170183
; 104302690
; 106228658
; 117488203
; 117498461
; 121362602
; 124671348
; 124883381
; 124883382
; 124883384
; 125357054
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03MIR
- Formula
- C23H25N5O5
- Canonical SMILES
- COC1=C(C=C2C(=C1)C(=NC(=N2)N3CCN(CC3)C(=O)C4COC5=CC=CC=C5O4)N)OC
- InChI
- 1S/C23H25N5O5/c1-30-18-11-14-15(12-19(18)31-2)25-23(26-21(14)24)28-9-7-27(8-10-28)22(29)20-13-32-16-5-3-4-6-17(16)33-20/h3-6,11-12,20H,7-10,13H2,1-2H3,(H2,24,25,26)
- InChIKey
- RUZYUOTYCVRMRZ-UHFFFAOYSA-N
|