| Cross-matching ID |
- PubChem CID
- 5388962
- PubChem SID
-
9191
; 7979157
; 11112847
; 11335583
; 11360822
; 11364201
; 11366763
; 11369325
; 11372634
; 11373494
; 11377487
; 11461794
; 11485085
; 11489271
; 11491293
; 11491823
; 11495121
; 14779899
; 14877707
; 17185069
; 29214998
; 39404326
; 46507920
; 47365119
; 47588933
; 47588934
; 47810682
; 48110391
; 48184936
; 48415941
; 49698433
; 50073899
; 50103938
; 50103939
; 53787340
; 57362754
; 75931775
; 89736144
; 92309084
; 93166909
; 96024588
; 99313640
; 103179319
; 103928457
; 113955320
; 127931871
; 134358398
; 135012773
; 137100856
; 140098266
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00SEB
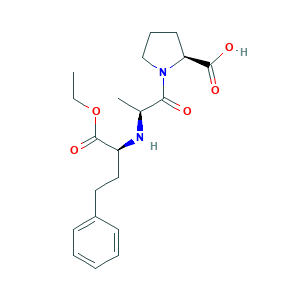
- Formula
- C20H28N2O5
- Canonical SMILES
- CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2CCCC2C(=O)O
- InChI
- 1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1
- InChIKey
- GBXSMTUPTTWBMN-XIRDDKMYSA-N
|