| Cross-matching ID |
- PubChem CID
- 21319
- PubChem SID
-
13913
; 592659
; 8165699
; 11406647
; 14833460
; 15478401
; 29288717
; 46508276
; 47206142
; 48416015
; 50064858
; 57304807
; 57330858
; 77367777
; 81065485
; 103510783
; 104133797
; 104352937
; 117541883
; 124766224
; 134338408
; 134984533
; 135968014
; 137003738
; 137272193
; 160849692
; 160963649
; 162173005
; 163413556
; 163848983
; 175442217
; 179316780
; 196392852
; 198978091
; 223557234
; 223672511
; 226395928
; 251912553
; 251916662
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Q2AT
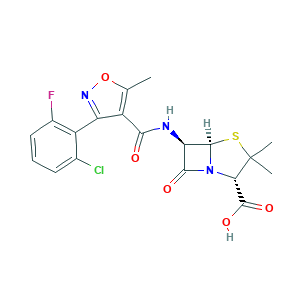
- Formula
- C19H17ClFN3O5S
- Canonical SMILES
- CC1=C(C(=NO1)C2=C(C=CC=C2Cl)F)C(=O)NC3C4N(C3=O)C(C(S4)(C)C)C(=O)O
- InChI
- 1S/C19H17ClFN3O5S/c1-7-10(12(23-29-7)11-8(20)5-4-6-9(11)21)15(25)22-13-16(26)24-14(18(27)28)19(2,3)30-17(13)24/h4-6,13-14,17H,1-3H3,(H,22,25)(H,27,28)/t13-,14+,17-/m1/s1
- InChIKey
- UIOFUWFRIANQPC-JKIFEVAISA-N
|